photography and
cinematography was a french invention.
first photo in history taken in a camera. exposure: 8 hours !

joseph nicéphore niepce
1826 photography with asphalt
THE FATHER OF PHOTOGRAPHY: the french joseph nicéphore took in 1826
the first fixed photograph in a camera obscura with a lens made by the french optician chevalier. EXPOSURE
TOOK 8 HOURS. the view is of his window. he worked 10 years to obtain that result. he coated a 8x10 inches metal
plate with asphaltum which is light sensitive. he called his method: "HELIOGRAPHY" (sun printing) he
died in 1833 at 68.

it was charles Chavalier who introduced joseph Niepce
to the painter Louis daguerre. they became friends and joined efforts in their
invention of photography.
they both experimented with the use of silver. Daguerre later found by himself
a process with a silvered copper plate made light sensitive with iodine vapors, silver iodide was formed, which he
discovered by accident the image could be developed with fumes of mercury. this way it was 20 times more sensitive to
light than asphaltum. it became the DAGUERREOTYPE.
 in 1847
in 1847
niepce made the important invention of photography on glass and the albumen
process.
he coated glass with egg albumen and potassium iodide, dried it and immerse it in silver nitrate bath,
exposed it in a camera for 5 minutes at a
lens aperture of f16 in a clear day under bright sunlight and developed a negative for 2
hours in a gallic acid solution.
this process for negatives in the camera was too slow and could not compete with the daguerreotype
because retarders in albumen keep the grains from growing large and sensitive. but no other medium than egg albumen
gave the finest silver grain in the history of photography.
1840 albumen paper

In 1849 the frenchman ( 1802-1872 ) Blanquard Evrard
made albumen paper for positives, he used kitchen salt instead of iodide. it was exposed in contact under a negative
for several minutes, the image printed out without development. it was fixed in sodium thiosulphate bath which washed
away only the unused silver salts. the image was toned in a gold bath to make it permanent.
for 50 years it reigned supreme for positives on paper and diapositives on glass, it
was displaced by gelatine papers. it was produced industrially until 1930.
LOUIS DAGUERRE
1838 photography with silver iodide

Daguerre got a pension for life from the french government who gave
the invention for free to the world. since niepce was dead, it was his son who also got a pension. 500.000 daguerreotypes were made in paris in 1838. it was practiced in the whole world until about 1850 when
it lost favor to the superior wet collodion process which was much more light sensitive and on glass could produce a
negative that gave of unlimited number of positive prints. daguerre died in france in 1851, 63 years old.
Ducos de Huron 1869 First color photography
 the french
the french
Ducos du Huron
in 1869
published in his book
"Les coulers en photographie, solution du próbleme"
his ideas he patented 1 year earlier.
with a multilayer of 3
light sensitive potassium bichromated
gelatine made with 3 colors:
yellow, cyan and red,
these were superimposed on each other. he produced in 1877 The First color photograph in history.

view of saint capris cathedral in south of france. ducos du huron
took this view from his room in the city of angens where he died in 1920.
the lumiere brothers 1907 color Autocrome process:
 THE TRIUMPH
THE TRIUMPH
Of Photochemistry !
Autochrome was invented by the french lumiere brothers.
IT was the first commercial color photography process to be taken directly in the camera.
it was based on one of ducos du huron ideas that he had disclosed in 1868.
autocrome was made with 3 black and white panchromatic gelatine silver bromide emulsions coated separately on the same
on glass.
under Each emulsion was coated a layer of stained potato starch grains which acted as filters. it was produced until 1935. it was the first colour process and the most beautiful photographic process ever invented !
Lumiere brothers Autochrome 1907
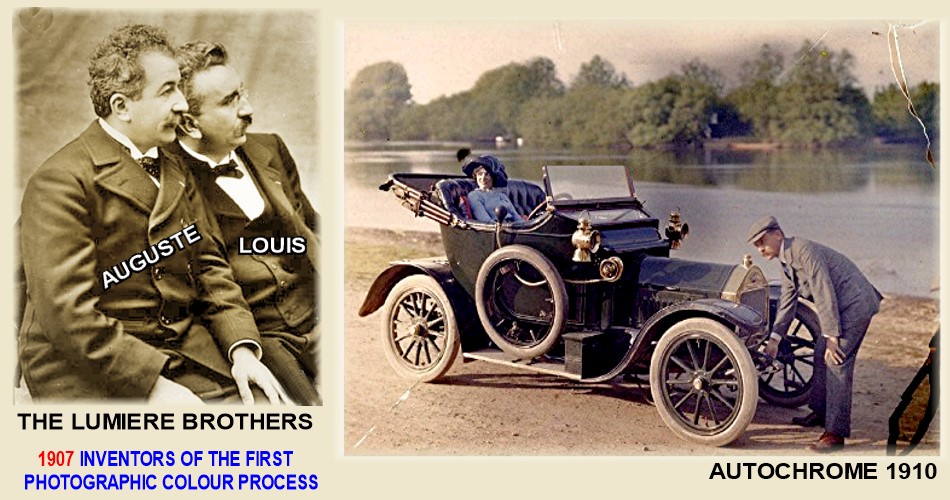
Lumiere brothers were inventors by nature. they invented engines and many things and
were also film directors. manufacture of autochromes was very expensive. exposure was
about 30x that of normal panchromatic plates. under sunlight it required one second exposure. By 1913 Lumire factory was making
6,000 Autochrome plates a day.
Antonio Lumiere, their father, had founded a glass plate photo factory in france that
became the largest in europe. in the museum Albert
kahn in france 72.000 autochromes can be seen. the Autochrome process
dominated the market for colour photography all over the world for nearly 30 years.
in 1935 it was replaced by processes like kodachrome.
Louis le Prince 1888 invents Cinematography
 the frenchman Louis le Prince invented motion
pictures 2 years before Edison invented his kinetoscope.
the frenchman Louis le Prince invented motion
pictures 2 years before Edison invented his kinetoscope.
Prince was a painter artist and chemist.
a pupil of photography of Daguerre.
he filmed (the first motion movie ever invented) in october 1888 which still
survives today.
he used silver bromide gelatine emulsion coated on strips of paper.
he made the single lense camera himself which he patented in england in november 1888.
he mysteriously disappeared and was never found.
France has the Lumiere brothers as the inventors of cinematography. the truth is Le Prince was the first inventor, but it was the Lumiere
brothers who elevated it to a professional perfection as we kow it today. his secrecy of his invention denied
him the rank he deserves in the history of photography.
Thomas Edison
and william kennedy 1891 kinetoscope.

in 1892 Thomas Edison copied the
principles of Prince and he and his employee william
kennedy made the kinetoscope for cinematography. the image on light sensitive silver
gelatin emulsion was not projected and only could be viewed through a peephole and only be shown to one person at a
time. this was a 500 kilogram machine that required a studio to be shot, and could
not be taken outdoors and showed no natural motion.
Edison sued a competitor in a court of law of
united states claiming he was the "sole" inventor of motion pictures. after analisis of all evidence, the
judge ruled in that case that Edison was not the inventor that he had only combined two
earlier inventions of european origin. many documents still today wrongly adscribe this invention to him.
The brothers Lumiere 1895
Revolutionized Cinematography.

they invented a camera called the cinematograph that could record, develop and project a 35
mm perforated wide film of silver gelatin emulsion film in general use then. a quiet machine, very light about 9
kg, (more than 50 times less weight than Edison's camera) and portable, 16 frames per second, hand cranked. much
superior in performance and properties to Edison's bulky and very limited
apparatus.
THE WET COLLODION PROCESS 1855 - 1880
 the french
the french
Gustave Le Gray
theorized the collodion process in 1850.
but it was the englishman
Frederick Scott Archer
who invented (in 1848) and published in 1851 the
new practical process of wet collodion. both Archer and Le Gray were artists and sculptors. they did not know each other.
by the end of 1860 wet collodion had entirely
replaced the daguerreotype which produce a positive image that could not be
replicated. daguerreotype was an expensive method and very toxic because it was developed with mercury fumes. collodion
a transparent colorless varnish, dosed with potassium iodide or bromide can be soaked in a solution of silver nitrate
while wet and exposed inmediately in a camera behind a lense. coated on dark colored metal it gave by reflected light a
direct positive ( the tintype).

if coated on transparent glass it gave a negative by transmitted light and 3 times more
sensitive to light than daguerreotypes.
Archer did not patent his invention, he died in extreme poverty
in england at 44 years old in 1857.
dry collodion plates and emulsions were made by many but it could not equal the sensitivity of the wet plate.
the wet collodion made a negative on glass,
this could produced an unlimited number of positives
usually made on light sensitive silvered albumen paper. it gave lines of unequaled resolution even to this
day.
Wet Collodion
1855 - 1880 photography with silver salts
 was the negative process from the year 1855 until 1880 when it was displaced by gelatine silver bromide.
was the negative process from the year 1855 until 1880 when it was displaced by gelatine silver bromide.
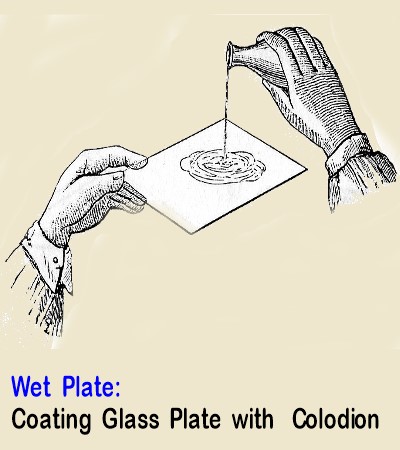
To work the Wet collodion process was not easy; the glass plate was coated with a collodion varnish dissolved in ether
and alcohol,
this clear synthetic resin varnish served as a vehicle for the silver salts.
Coating of the glass plate was done in the darkroom about 5 minutes before it was required for use.
For exposure, the plate had to be placed in the camera and exposed while the recently coated and sensitized
collodion film was still wet,
otherwise if the coating dried, the collodion pores would close and loose sensitivity as well as repelling the
developer solution. All had to be done very quickly at a known pace without interruptions because the silver salts
started loosing and changing sensitivity immediately after coating.
A change in sensitivity could mean a wrong exposure time, making the plate useless. Portrait photographers worked in
studios with large windows to allow in the maximum amount of daylight, the lens was often opened to its maximum
aperture, exposure under this conditions ranged between 2 to 10 seconds.
In the open, landscapes were taken with lenses set at medium apertures for sharpness with
exposures of about 5-10 seconds under good sunlight. The average wet collodion plate at its peak of its short
lived light sensitivity has a speed equivalent to ASA or ISO
0.25 (10 degrees Warnerke). all cameras had the rapid Petzval lenses.
 All workers of the epoch were influenced by this process which used silver iodide,
All workers of the epoch were influenced by this process which used silver iodide,
as its main sensitizer (not bromide).
In compounding the sensitive mixture Silver nitrate was used in excess over the halide salt which was potassium
iodide,
and development was effected in an acid medium.
THE TINTYPE
1853 the popular process of the masses

born in paris in 1824 died 1896,
the french Adolphe Alexandre Martin professor of chemistry, invented the ambrotype 1852, and the tintype or ferrotype in 1853. the processes he gave free to the
world. He published his method in the lumiere magazine and the academy of
sciences. he built the world's largest mirror at the time of the paris observatory telescope. he also made lenses.
in the tintype wet silvered collodion was coated on thin and dark sheet of
metal, underexposed wet or dried in the camera and the negative produced because of the dark ground on the metal sheet
gave a direct positive. the tintype was the rage of the working class it
only costed 10 cents of US dollar compared to a daguerreotype which costed 5 dollars, a week's wages then. it
was patented by others in the united states and in england in 1856. it was called at
first " melainotype " then the " tintype " and
finally the " ferrotype ".
 the tintype in a thin sheet of iron and unbreakable replaced the
ambrotype in 1865. ambrotype: invented in 1852, took over the daguerreotype by 1860. the ambrotype was wet collodion coated on glass, it gave a negative but the glass coated in the back
with a black varnish gave a positive image. compared to daguerreotype it was dull
and the glass could brake.
the tintype in a thin sheet of iron and unbreakable replaced the
ambrotype in 1865. ambrotype: invented in 1852, took over the daguerreotype by 1860. the ambrotype was wet collodion coated on glass, it gave a negative but the glass coated in the back
with a black varnish gave a positive image. compared to daguerreotype it was dull
and the glass could brake.
the tintype in 1940 costed 25
cents of a US dollar and it was made and sold in the open air, by photographers
working in booths, fairs and carnivals, and itinerant sidewalk photographers.
the ferrotype or tintype could be taken
and finished in 7 minutes. lost ground to a higher quality positives on paper
made in silvered egg albumen prints. it lasted for almost 100 years.
WORKING THE WET COLLODION
 the silver halide salts employed was silver iodide,
the silver halide salts employed was silver iodide,
which embedded in the collodion with an excess of silver
nitrate (not bromides) was developed immediately after exposure in a solution of Gallic acid with acetic acid (or pyrogallic acid).
The excess of silver nitrate on the coating was used here,
the Gallic acid Re-depositing it on the latent image.
( Physical Development ).

the subject could not move for 10 seconds
which was the exposure of wet plate collodion plates. wet collodion due to its high
speed and low cost, had displaced the Daguerreotype, An expensive process
reserved for the rich and higher classes. Wet Collodion gave the working classes a
low cost portrait for the first time in the history of photography. Collodion
coated portraits on tin plates were sold by the millions
world wide. These were called Ferrotypes or tintypes. Wet collodion process still stood unrivalled in the year 1875. Landscape photographers prepared wet collodion plates inside dark tents. The
resolution given by these plates were never again equaled by other processes.Just as the wet collodion process itself could not equal the resolution on the positives of the older
albumen process on glass.
THE GELATINE PROCESS
1868: Harrison the silver gelatin process was an
English invention
W.H. Harrison published in the British journal of photography of
January 17, 1868, an account of his experiments with gelatine, cadmium iodide and
cadmium bromide. He coated glass plates with this emulsion which he developed with alkaline pyrogallol. He
said that the image developed quickly and appeared with such a great intensity that he had
never seen before with collodion, but was of no use because of its rough uneven surface. (Not enough
gelatin in proportion to the silver salts, left a rough matt surface) He recognized
this and on increasing the gelatine content, did not get an image.
This failure of Harrison discouraged other workers for many years. The fact that he
developed with alkaline pyro proves that the cadmium bromide was correctly in excess over the silver ions as
it should be. Many years. Later, Harrison in his old age, recalling his failed
experiments remarked that he did not get an image because he probably forgot to add the pyrogallic acid to the
developer. An omission that denied him the glory bestowed instead on Maddox.
Maddox 1871
Richard Maddox has been called by historians the
inventor of gelatine silver bromide emulsions because of directions He published In September 8, 1871 in the British Journal of Photography
under: experiments with Gelatine-Bromide. He used Cadmium bromide with an excess of silver nitrate and physically
developed with acid pyro. Nevertheless he used the right proportions of gelatine to give a smooth surface emulsion.
He did not realize the emulsion had to be washed and that
the bromide had to be in excess of the silver. His emulsion, with excess silver was extremely
insensitive; with an acid physical developer in the presence of gelatine, it
could not approach a fraction of the sensitivity of the wet collodion plates.
Furthermore with free silver in the coating the plates had no stability whatsoever, they would not keep. On top of
all those faults the intensity of his developed images were so weak that he had to intensify them with adding
more free silver to the developer. But his experiment greatly encouraged other workers.
Gelatine EMULSION WASHING
King and Johnston 1873. It was J. King, November 14 ,
1873 (British Journal of Photography) who first introduced the washing of the digested emulsion.
Expulsion of soluble halide salts out of the emulsion mixture, the washing was effected by a very slow process of
dialysis. J. Johnstons method was better, He worked by setting the gelatine
in the cold, breaking it into small pieces and washing it in very cold water to get rid of the great excess of
soluble bromide (which hinders maximum light sensitivity) as well as the soluble potassium
nitrate. Also, the washed coated emulsion gains enormously on stability because the soluble nitrates do
not crystallize on the glass plate as they do when left in the coating as when the emulsion is not washed.
Johnston 1873 In 1873, (also in November 14)
recognized the fact that silver gelatine emulsion should be formulated with an excess of halide, (instead of
excess of silver as published by Maddox earlier) that is, the bromide should
predominate, This was of Extreme Capital Importance, it set all other
workers in the right path for making practical high speed emulsions. He also recommended as J. king had done in the same issue of the Journal, that the emulsion should be washed to get
rid of the excess of the soluble salts. Johnston had also discovered independently
just as king had, that washing out excess salts was beneficial. Burgess 1873
A few months even before the J. Johnston and King publications, In
July 1873, The Englishman J. Burgess offered for
sale in London in a low scale, the First Gelatine Silver Bromide Emulsions.
Burgesss gelatine emulsion was by far not as sensitive as
the wet collodion plates who reigned as the negative process of the day. He never disclosed his formulas.
His secrecy denied him a higher rank in emulsion history. But he correctly used bromide in excess because he
developed with alkaline pyro developer. He was far ahead of Maddox in this respect,
also his emulsion was stable. Maddox had used pyrogallic acid in a acid medium to
physically develop his gelatine emulsion formulated with excess of silver nitrate.
Gelatine Pioneers 1850 - 1853
Many had tried to use gelatine as a binding agent for silver salts. (Poitevin in 1850,
Gaudin in 1853) Most experiments failed because it took a long time to realize that silver bromide (not
silver iodide) must form the principal ingredient in such an emulsion and furthermore that the great light
sensitivity of silver bromide becomes effective only: with alkaline chemical
development.

Ferrotype or TinType
wet collodion portraits on brown or black Plates were the rage for more than a generation.
Even by the 1940s
they were
still in production by itinerant photographers every where. Only in the Tintype the Collodion process wet or dry refused to die.
 1871 Maddox
1871 Maddox
the gelatin process
According to jose maria Eder who is a historian himself,
Maddox sent him in 1900 brown color delicate
landscape negatives on glass produced with his process at the time.
But History has to be re written. It should read:
Gelatine Silver Bromide was invented by Maddox, Johnston and King.
Maddox has been given much more credit than he deserved probably because of his fine
human qualities. He was an unassuming very humble person ( a doctor by profession) who gained the sympathy of all who
got to known him, including publishers and historians.

J. Johnston
set the Gelatine to form a jelly and washed emulsion under water.
This was a Great improvement !
A washed emulsion usually
doubles or triples
in speed after washing.
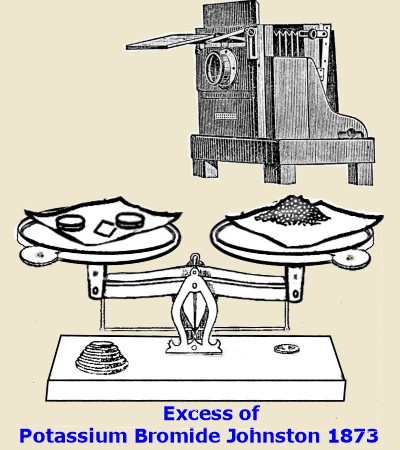
170 parts of Silver Nitrate combines molecularly exactly
with 188 parts of Potassium Bromide, Johnston in 1873.
added much more than 188 parts of the bromide, to leave it in excess over
the silver.
This produced no fog in alkaline development with extremely more sensitivity.
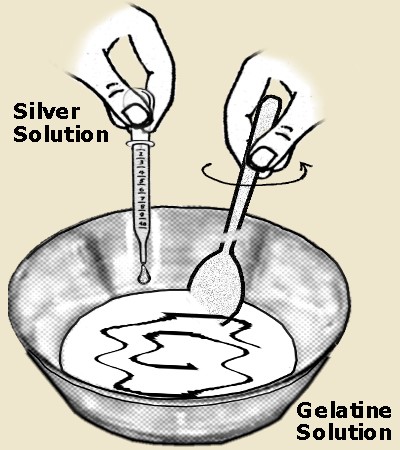 Bolton
Bolton
emulsified with the potassium bromide dissolved in a 1- 2 % gelatine solution
in which the silver grain grew larger and more sensitive.
Bolton 1873
emulsification with little gelatine.
In 1873 W. B. Bolton, publish his method of
emulsifying with very little gelatin at first during the first stage of cooking
and later adding the rest of the final gelatine content to the emulsion. With this technique which was used for more than 125 years, emulsion sensitivity increased at least 100
%.

sensitivity 1855 to 1877
of gelatine silver bromide emulsion.
The fastest gelatine silver bromide emulsion could only equal the sensitivity of wet collodion plates but could
not surpass it.
Gelatine plates were used only by a very few landscape photographers and almost
exclusively in England. In 1877 a portrait of the Pope Leo XIII, posing in the
Vatican gardens was taken with a gelatine silver bromide plate with an exposure of 1
second. This caught the worlds attention and greatly promoted adding new energy to the new gelatine
process.
Bennet 1878 introduces First EXCESS OF KBR. Charles
Bennet published in March 29, 1878, (British journal of photography) that
gelatine silver bromide, with excess of potassium bromide, gains greatly in sensitivity when heated for about 4 -5
days at 32 c. The gelatine of course broke down and decomposed by fermentation
due to heating for such a long time, But by the use of Boltons method which
involved replacing the fermented gelatine with fresh one at the end of the ripening process, the road was opened to surpass the speed of wet collodion. A great challenge at this time.
 Mansfield 1879
Mansfield 1879
Improves Ripening Bennets emulsion heating
technique was soon improved, by Mansfield, instead of many days at 32 c, the freshly mixed gelatine emulsion
was submitted from 2 to 12 hours at 60 to 80c, or half an hour near
boiling point or heated for a short time in a boiling water bath.
This opened ( first in England )
the commercial production of gelatine dry plates...
Surpassing finally for the first time in 1878-1879 the speed of the Wet
Collodion plates this manner of making emulsions by high temperatures some times in the presence of acids; became
known as the boil or acid method distinguishing them from emulsions made at much
lower temperatures and with alkalis like ammonia or sodium carbonate.
With the Bennet / Mansfield Method of heating the
emulsion from 2- 12 hours at 60-80c after precipitation Great light sensitivity is attained because the silver
grains grow larger forming colonies. This basic step is used this very day to get speed.
 Gelatine silver bromide emulsion
Gelatine silver bromide emulsion
coated on glass
first begun to be
introduced as
an article of commerce
in 1878,
4 times faster than
the Wet Collodion Plates.
About ASA 1.5
1878 Commercial Gelatine
Silver Bromide Plates ASA 1. 5
For the so called instant photography however, that is for a hand held camera exposure without a tripod, a sensitivity of 19-21 degrees Warnerke was required, which was about 2- 3 ASA.
1878 first commercial gelatin plates

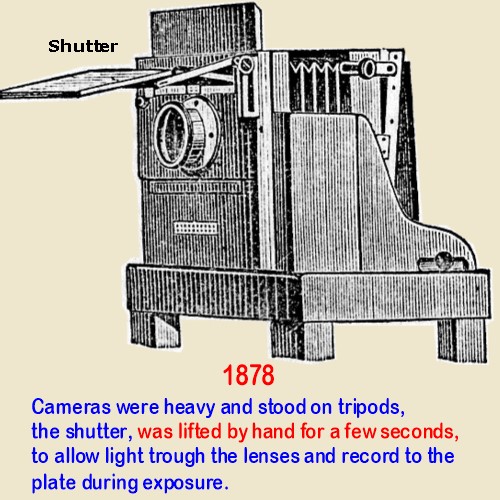
Wet
collodion plates
Had a Sensitivity of 10 Degrees Warnerke ( 0.25 ASA )
The ideal in the 1880s was to Reach the Sensitivity Shown on the Highest Number
on Warnerkers Sensitometer, which was 25 degrees,
or ASA 8, Today. In 1878 Plates were slow and mechanical
shutters being unnecessary had not been invented,
Hand held cameras cameras did not exist. With the necessary long exposures, a hand held picture would not be sharp.
Moving subjects were also impossible, they would record blurred.
Eder 1880, introduces Ammonia
Method Reaching Easily ASA 3 In 1877, again, J. Johnston described the use of ammonia for ripening gelatine silver
bromide emulsions; the process was improved by Monchoven who made the important discovery that the growth of silver
halide crystals was greatly accelerated by ammonia. Larger grains meant more sensitivity and shorter exposures
times.
 But who really perfected the silver oxide ammonia process bringing it to a
practical success was Jose Maria Eder in
1880.
But who really perfected the silver oxide ammonia process bringing it to a
practical success was Jose Maria Eder in
1880.
(in the Viennese Journal Photographische
Correspondez) Eder published his method of adding ammonia to the silver solution until the brown precipitate of
silver hydroxide is re dissolved to give a clear solution, and adding this to the gelatin containing the potassium or
ammonium bromide to make in a very short time, and the simplest of manners; a high speed high contrast emulsion.
This emulsion was so rapid and clean that when coated on glass plates it could be used in the camera for negative
work, or it could be coated on paper to yield very rapid enlarging bromide paper of the highest quality for positive
work.
To avoid fog, He correctly identified 40c as the safest and best temperature for
making ammonia emulsions. Since this process, as said earlier, yielded rich, high density, clear high contrast
silver bromide emulsions suitable for negative or positive work and for portrait as well as landscape
photography,
With a Speed of ASA 3, a Front Lit subject under Bright Sunlight can be taken
with a Shutter Speed of 1/30th of a second with a lens aperture set at f 5.6 it is
a practical Emulsion Speed for Negative work.
 it was the miracle every
it was the miracle every
one was waiting for.
Even today is the less expensive, the more rapid, the easiest and the more certain way of making a good emulsion for
beginners. The boiled type of Bennet emulsions of that time, although of good quality lacked the contrast, density
and brilliancy required for positive bromide paper or photomechanical plates.
The method as worked in 1880 by the Bennet process
(with out ammonia) which were later to be known as neutral acid or boiled emulsion process was not yet at that time
suitable for producing an all around emulsion.
Soon after the publication by Eder
of his reliable ammonia, quick and easy method of making rapid emulsions and that any one could use for free,
(Eder never patented his inventions he gave them unselfishly to the world).
gelatine plate factories as well as paper factories, sprung first in Europe and later all over the world.
About the same time Eder also invented gelatine silver chloride and silver
Chloro-bromide emulsions for development. These soon displaced albumen paper for positive
work. The road was now paved for the launching of a gigantic new industry as the world had never seen
before. IT LASTED 125 YEARS until displaced by digital photography !
The 1880 Gelatine Dry Plates
Made by Eders Ammonia Process Had a Speed
of about 21 Warnerke (ASA 3) 7 times faster than wet collodion plates!
Die Moment Photographie Instant Photography Had Just Begun!
With Landscape Lenses exposures could be made as Short as 1/20th of a Second and with
Faster Portrait Lenses as short as 1/50th to 1/200th of a Second.

Front lit subjects / bright noon sunlight Cameras were to be soon Re designed and shutters had to be improved
because the new gelatine process allowed for hand held photography for the first
time since the invention of photography in 1839. (Daguerreotype).
The wet collodion process was forced into retirement. It disappeared
completely in 1880. (except for tin typists) It had served the world well for 25 years ! It had given
to Science, as well as to Art and the working classes, -its first portraits.

Hand held Camera
exposures
beginning in 1880
were now possible.
ASA 3 , allowed
1/50th to 1/200th
of a second
shutter speeds
that stop motion
for the first time.

first kodak camera in 1888
Had no viewfinder.
The shutter speed was
fixed at 1/25th
of a second
and had no manual controls.
Slogan was:
You Press the Button We Do the Rest
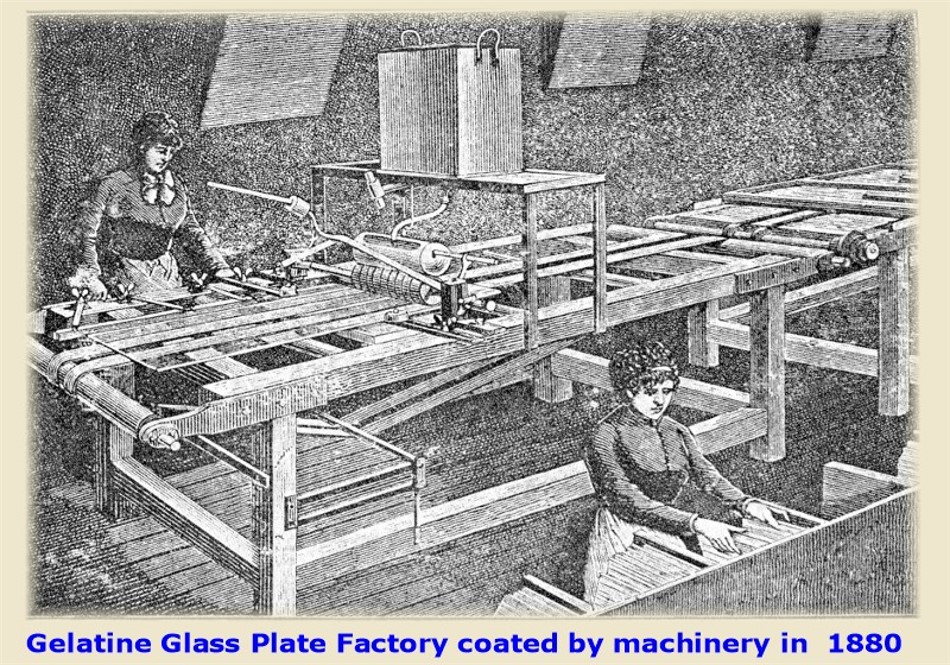
Gelatine Emulsion Glass Plate Factory being coated by machinery in the 1880s. Glass plates were coated by hand in
most factories well into the1900s. An experienced worker could hand coat about 2000
glass plates in a days shift. The new industry promised so much fortune that publications of new formulas
stopped appearing.Methods and formulas were kept jealously guarded
secrets from 1880 on !

1890: ASA 6-8
From 1880 to 1890 instant plate speeds about doubled. They were in 1890 about 24 to 25 degrees Warnerke (6 8 ASA)
Much before this time, most publications about emulsion had stopped appearing in journals, improvement of the above
mentioned techniques and discovery of new ones were being held as closely guarded secrets in the new successful emulsion manufacturing trade.
1900: ASA 8-10
In 1900, Eastman Kodak introduced its Extra Rapid Plates claiming to be 30 Warnerke (ASA 25) Eder himself measured
its sensitivity and found them to be only half of the claimed sensitivity, that is: 25-26 Warnerke (8-10 ASA) or 125-160 H&D. Many, if not most, manufacturers inflated their speed values
to increase sales. Inflation of speeds values were practiced until beyond the year 2000.
1915: ASA 16 - 20
By 1915, speed had about doubled again, due to a good choice of gelatins,
higher physical ripening temperatures, the use of the optimum amounts of potassium iodide which permitted to heat
the emulsion at higher temperatures and for a much longer time to gain more speed. Potassium iodide greatly
protecting the silver bromide from fog. Also, just plain good practice acquired by experience was producing emulsion sensitivities of about 16 ASA or
ISO (about 260 practical HD) 28 Warnerke. The old Warnerke sensitometer to measure
plate sensitivity was no longer adequate. Its highest values: 25 (ASA 8) had already been surpassed
Gelatine Silver Bromide Orthochromatic plates 1882
Ordinary gelatin silver bromide and silver iodide grains are natively sensitive to blue violet radiations only.
Emulsions stained with Eosin were called orthochromatic or
isochromatic.
In 1882 Attout
and Clayton took out a French patent for the orthochromatization of gelatine silver-bromide plates by the
use of Eosin as dye.
Vogel: dye sensitization
working with the wet collodion process, had discovered in 1873 that the silver
grains could be stained with dyes (Eosin) to obtain color correct negatives when
photographing color objects.This, (later called spectral sensitization) sensitized
monochrome emulsions to see green and yellow colors as the human eye sees them.
1884 Superior Orthochromatization with ERYROSINE.
In 1884 Eder again introduced a superior red dye Erythrosine (potassium salt of tetraiodo-fluoroscein) to stain the grain of gelatine silver
bromide emulsions and make them orthochromatic. Due to the increased sensitivity to artificial light like candle,
gaslight, incandescent bulbs, this plates permitted much shorter exposures than
ordinary plates in everyday photography and are still considered the best in its class. Erythrosine is an acid dye of the Eosin group. A basic alkaline type of dye is the
" Cyanine "group.
Furthermore Erythrosine or Erythrosine is a dye
easy to obtain even today. It is used for microscopic work in all
countries. It makes the silver grain see green ( foliage / vegetation etc) and some orange to yellow
radiations.
The extra bluish variety of erythrosine sold for microscopic work today , sensitizes also for green but a lot more
for yellow. To better differentiate greens and oranges in the original scene, stained silver with any type of
erytrosine can be exposed through a yellow filter, or a yellow dye (like tartrazine) added to the emulsion itself.
Tartrazine is a yellow dye easy to obtain (used for
food coloring) it washes out of gelatine rapidly.

Gelatine Silver Bromide Panchromatic plates 1902
Erythrosine plates while sensitive to green and yellow are not sensitive to red,
(this is an advantage because emulsion can be handled under red light) Cyanine
dyes sensitize silver grain to red. Erythrosine and cyanine can be admixed
together in the emulsion to make panchromatic emulsions which will see all the
colors in nature as the human eye sees them.
This general sensitivity to all colors is called panchromatization. But this mixture
does not work well together because Erythrosine is an acid dye and Eosin a basic (alkaline) dye. Better is a
mixture of Chinoline or Quinoline red and Cyanine which are both basic dyes. This mixture was called by
Vogel Azaline.

In 1902 Miethe and Traube
introduced Ethyl red as a panchromatic sensitizer, this dye made the
silver grains see from the yellow greens up to the dark oranges. It is a Cyanine basic
type of dye. Ethyl Red: C12 H8N2I ( note in formula Carbon Hydrogen Nitrogen
Iodine atoms) Chemical name: 1:11 diethylisocyanine iodide.
Super Sensitizing Cyanine Dyes 1926
When In 1902 Miethe and Traube introduced Ethyl
Red (a cyanine dye, isocyanine) as a panchromatic sensitizer, the story had just begun. The door was now
open for the production of Extra Light Sensitive panchromatic plates.
Ethyl Red by itself makes silver grain see all colors.


 1903 Even more powerful red sensitizing cyanine dyes, which allow photography even through
HAZE AND FOG, were introduced by Enrst Konig. Dyes like orthochrome T, pinaverdol,
pinachrome, pinachrome violet and Dicyanine.
1903 Even more powerful red sensitizing cyanine dyes, which allow photography even through
HAZE AND FOG, were introduced by Enrst Konig. Dyes like orthochrome T, pinaverdol,
pinachrome, pinachrome violet and Dicyanine.
the dyes made by konig not only advanced panchromatic still and cinematographic
emulsions, but also a permanent color photography process made with them was placed in
the market in 1903 by a german firm who employed konig, called: Pinatype. color images were very stable to light. exposed to direct sun for 1 year they
do not fade. pinatypes were made by firms, professional and amateur
photographers for 75 years. all formulas and methods konig disclosed in 1903 in
his book. " natural color photography "
1906 powerful red sensitizer Pinacyanol
Iodide
(a carbocyanine dye) was introduced by Homolka in 1906, Commercial plates were termed Panchromatic. ( Pinacyanol
blue is also a powerful sensitizer for Red / it is blind to green) Dicyanine is a
sensitizer for red and infrared, due to its tendency to produce fog in the presence of ammonia it can be replaced
with kryptocyanine or Neocyanine.
 josef max petzval in 1840
josef max petzval in 1840
a hungarian mathematical genius calculated in 6 months (assisted by 8 austrian mathematicians) the construction of
a portrait lens (f/3.6) that,
compared to the french chevalier lens used in daguerreotype cameras in 1839, reduced exposures 60 times allowing portraits of
people to be taken in daylight in 30 seconds!


in 1855 about 8.000 of petzval lenses were used in
cameras working the wet collodion process. by 1862 opticians like
voigtlander had produced 60.000 lenses.
without it no large format photographic process would serve well. the lens is still used today in big image
projectors. in 1925 came Neocyanine
(Kodak) Rubrocyanine 1928, Allocyanine 1929,
(IG.Farbenindustrie). And the Thiacarbocyanines, (more powerful than Pinacyanol ) were discovered by Hamer in 1928.
The era a high speed films had begun.
All of these
Orthocromatizing dyes Eosin, Erytrosine, Ethyl Red etc and pancromatizing dyes increased the primitive
sensitivity of the emulsion to daylight about 100 %.
For Infrared:
Xenocyanine (Brooker,1931) dicarbocyanines (1932, Konig.W) Tricarbocyanines (1933 Fisher and Hammer)
Tetracarbocyanines and Pentacarbocyanines (1935, Brooker and Keyes)
Merocyanines for green (1935 Kendall and Brooker) and
many other classes of acid and basic cyanines.
 Super Sensitization
Super Sensitization
was a breakthrough. It was discovered that when certain combinations of two different dyes were used a secondary
dye could extend the silver grain sensitivity of the primary dye by 3 to 25 times!
This was called Super Dye Sensitization. In real practice however the emulsion
gain in sensitivity, would rarely be more than 200 % and it took some years for these emulsions to reach the
market. All of these dyes, (and many more) as emulsion workers learned how to use them, alone or with one another,
and in combination with new emulsion precipitation techniques, and other advancements like Sulphur and gold
sensitization started pushing, a few years later, beginning around
1935, panchromatic emulsion speed beyond ASA 125 !


Sheppard
Sulphur Sensitization 1927 ASA 32 !
In 1927, ordinary emulsion speeds were at least doubled when Sheppard of the Kodak laboratories discovered Sulphur sensitization.
He found that Sulphur
complex compounds were responsible for the super light sensitivity imparted to the silver by some gelatines.
These sulphur compounds were naturally found on all types of gelatins, in greater amounts in what later was to
be called active types than in the so called inactive types. By adding controlled amounts of complex sulphur compounds to the emulsion
after or before washing and heating for a while... emulsion speed was at least
doubled. This was a sort of sulphur toning of the unexposed silver crystals which would then were able to
"see" more light.
 sulphur
helped to
sulphur
helped tobring emulsions in 1930
to the speed of 525
practical HD. (32 ASA)
Sulphur sensitization can be effected today simply by adding minute
amounts of ammonium or potassium thiosulphate to the emulsion.
Which by the way was always naturally present in the gelatins in varying amounts and ironic enough is also a part of the darkroom chemistry because this salt is non other than the ordinary Fixer used for processing the sensitive material.
Luppo Cramers Emulsion Precipitation Techniques 1930
Gelatine emulsions after mixing the silver salts with the alkaline halides, are kept for a while, before washing,
at temperatures anywhere from 40c (Ammonia emulsion) to 80c: (neutral emulsions) during this time the silver
crystal gains sensitivity, this part of the Ripening process or digestion before
washing is called physical ripening because the silver halide crystals grow physically in size. (Oswald)
 During the years
During the years
1927 - 1930 Luppo Cramer
showed that the emulsion after washing and re melting could be submitted to a Second
Heating or Ripening gaining still more sensitivity. This second ripening
or digestion was later to be called chemical ripening because the crystal in the absence of soluble bromides
is able to absorb sensitivity accelerating or retarding organic compounds also naturally present in the gelatins
(like the Sulphur compound Sheppard had
discovered)
During the mixing of emulsions, the action of adding the silver solution to the bromides dissolved in the gelatine solution is called Precipitation. Up to about 1930, the usual form of precipitation mostly in practice, was simply adding the silver solution in a fine stream usually from 1 to 10 minutes and with attention only to agitation, low gel concentration (Bolton) for more speed (about 1- 1.5 % gel) and temperature of the mixture.
Luppo Cramer showed that by long precipitation times (15 to 20 minutes) with or without ammonia, the crystals could be made to grow considerably obtaining thus more sensitive emulsions than by the usual process. This discovery along with the second ripening was FAR REACHING . It is true that some advanced manufacturers around 1928 were already SECRETLY using this technique, but it was Cramer who first published this technique with great detail making it generally known. His publications were supported by contrast, grain size and sensitivity curbs connected to precipitation times.
It is now known that all major negative
emulsion manufacturers in Germany adopted secretly Luppo Cramers technique and that each manufacturer further
improved it on their own. For example, almost all of them divided the silver solution in at least 2 parts. About 20
% to Half of the silver was added rapidly to the gelatine/ bromide solution, (this builds a crystal colony called
nuclei) and the remaining silver sol was added slowly and continually throughout a period of time that could vary
(according to speed, fineness of grain or contrast desired) between 5 to 30 minutes at 60 to 80c.
This second wave of silver bromide raining slowly on the first generation nuclei was capable of modifying the
grains and of producing extremely sensitive giant silver crystals (although of
much lower contrast and resolution)

1941 the german air force in world war 2 used luppo cramer technique to make
ultra rapid gelatine silver bromide emulsion for night photography. the precipitation time was 30 minutes. the
silver grain grew very large and very sensitive to light. this was the more rapid emulsion ever made up to the
time.
 At my research Lab,
At my research Lab,
with aid of Luppo Cramers method and other Techniques, with
an inert type of gelatine (sensitized with Sulphur and not panchromatic) I did work out a Superb ULTRA RAPID Negative formula that produced a speed of ASA 32 - 64 !!! by using all the tricks of the trade: "this
is the maximum speed possible without gold or dye sensitization ". SEE
IN CHAPTER 9, my emulsion number: 7, in this book.
1932 ASA 40-50 ( 24 - 25 Scheiner)
Luppo Cramers precipitation technique adopted by major emulsion factories was been improved upon with substantial
gains in emulsion speed. By 1935 all mayor emulsion manufacturers were already
producing or were about to begin producing panchromatic emulsions of extreme sensitivity, about: 2000
Practical HD ( ASA 125
1935 ASA 125 With Powerful Stabilizers or Antifogging Agents
The normal Emulsion stabilizer and antifogging agent added after washing and digesting the emulsion was Potassium
or Ammonium Bromide absolutely necessary to prolong shelf life on storage of the coated emulsion. But with such
extreme speeds, more powerful stabilizers than the mentioned above were being researched and introduced around this
time. They were highly guarded trade secrets:
Benzotriazole and 5-Nitrobenzimidazol Nitrate
were two of those agents and are two types that are easy to obtain and can be used as stabilizers for high speed
negative emulsions even today.
 Kolowsky 1936
Kolowsky 1936
Gold Sensitization
the hyper sensibilization of silver bromo-iodide emulsions with gold salts
was discovered by Kolowsky in 1936 at the Agfa
film factory in Wolfen. The process was of course kept secret for many
years.
but after the war it was disclosed. Gold Sensitization employed another salt long
used in the darkrooms, an alkaline gold toner made with gold chloride and an
alkaline thiocyanate, this allowed the speed of ordinary gelatine silver emulsions to be pushed 1 to 2 more
stops further, That is 100 - 200 % more speed and like sulphur sensitization without much grain size increase!
DOSAGE:
after washing, additions of up to 100 ml of a 1 in 10,000, solution of sodium chloroaurate per 1000 ml of emulsion
is recommended. After adittion submit emulsion to second ripening.
In combination with Sulphur sensitization, Gold sensitization pushed ordinary emulsion speeds usually much more than 2 stops
further. It could push an ordinary emulsion with a primitive speed of ASA 25 to ASA 100.
 All of
this was a blessing at the right time, For Compounding the New Line of Fine Grain Emulsions, because around this
time 35 mm film for miniature cameras was beginning to have a great demand.
All of
this was a blessing at the right time, For Compounding the New Line of Fine Grain Emulsions, because around this
time 35 mm film for miniature cameras was beginning to have a great demand.
35mm film was not taken Seriously by
Photographers until Around 1930. then the miniature Camera was no longer seen
as toy. With sulphur and Gold sensitization high speed with fine grain was obtained. Higher Resolution Emulsions
For 35 mm Film and Enlarging
Up to about 1925, most negative emulsions
had coarse grain and lower resolution namely because to obtain more speed
the precipitants were too concentrated,for example the bromides were concentrated at the order of 10 to 20%
or above, and the silver solution from: 10 to 30%. This made very large grains of lower
resolution, no one was complaining because the negative plates in use then were usually larger than 4x 5
inches.
CONTACT PRINTING 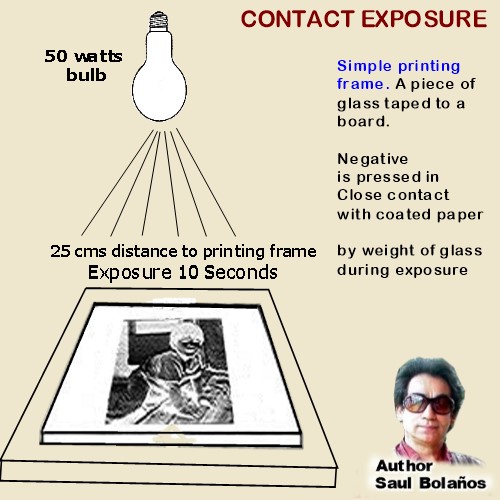 BEFORE 1950
BEFORE 1950
Most photographer just made contact prints or 2x enlargements.
One of the secrets for high resolution, was to adjust the formula for long precipitation times and vigorous
agitation (without ammonia) and adjust precipitants at no higher than 10% concentration. After washing,
emulsion was sensitized with sulphur, gold or dyes.
High Speed lower contrast emulsion were mixed together, after preparation, in adequate proportions with
contrasty higher density emulsion to get the desired speed and density curbs.
ENLARGING
AFTER 1960 very few photographers were making "contact prints" because
the negatives used were very small, instead the small negatives were placed in projectors and enlarged many
times the original size of the negative.
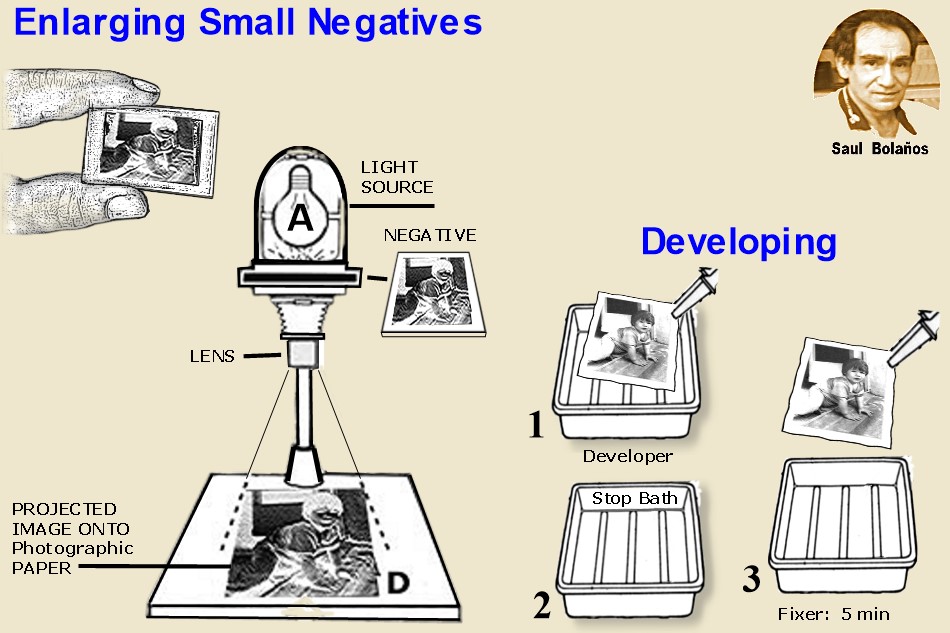
the projection was made usually into positive paper. this method wasvery
economical because large format films were more expensive but generally the quality of the prints suffered after
that time. Ordinary photo enlarger, throws image on base board at any desired size by adjusting the lens to
negative distance. manufacturers inflated the speeds of their material
Color negative and reversal positive emulsions rated by their manufacturers at ASA
25 (or ISO 25) in 1945 were actually half that speed, that is
ASA 12,5 But soon by 1949, Real speed on color films was reaching a fine grain ASA
64.
1945 Techniques Combined: ASA 320.
Add to gold and Sulphur sensitization an
optimum long precipitation time a la Luppo
Cramer with a second ripening, stain the silver grain with one or two super cyanine dyes to make it color
sensitive (ortho, or panchromatic) and by 1945 we had negative monochrome
emulsion speeds of about 320 ASA (or ISO)
1980 Ultra speed Negative Emulsions: ASA 1250
In 1970 Kodak rated its monochrome Royal x pan negative emulsion film
speed at 1250 ASA,
 in
enlarging The grain of ASA 1250 emulsions was too large,
in
enlarging The grain of ASA 1250 emulsions was too large,
Resolution was very poor. Kodaks emulsion known as " Tri-x " ASA 400 in
miniature 35mm roll films in the year 1975 and Agfa:
" Agfapan " ASA 400, and Ilford: " HP-5 "
ALL OF THESE these Black and white negative emulsions on celluloid on enlarging in projectors were very grainy.
 Resolution WAS ALSO VERY POOR and when
developed in a fine grain developer with a silver solvent, grain improved but resolution suffered.
Resolution WAS ALSO VERY POOR and when
developed in a fine grain developer with a silver solvent, grain improved but resolution suffered.
ASA 400 and
1250 films
could ONLY be satisfactory for some types of work. The first for Journalism and the second for surveillance, But
never for good quality general photographic work. Kodaks Microdol fine grain
developer dosed with a silver solvent to make the grain o their grainy black & white high speed films of ASA
400 less objectionable. Agfas ATOMAL developer did the same for their high
speed grainy films. These developers were in general use by photographers from about 1960 to 1995,
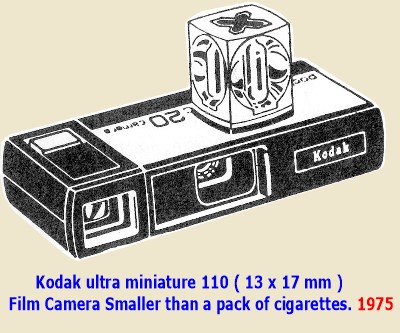 In 1975 the public was using
In 1975 the public was using
cameras with color films material (size 110)
even smaller than the miniature 27x 35mm which was employed in cinematography and by the bulk of serious
amateurs.
These cameras used fine grain
negative color film ASA 80, or fine
grain color reversal positive film ASA 64.
 Larger film format
Larger film format
( 3 x 4
inches)
that the general public
was still using in 1949
had disappeared for
general use by 1960,
(as did also glass plates).

only an extremely small minority were using film larger than 2 inches. These were photographers in studio and in
the advertising trade.
The ASA (or ISO) 125 color or black and white
negative films, were the last of the Real fine grain negative emulsions. The bulk of black and white or monochrome
negative emulsions being manufactured around 1975 ended up in multilayer
tri-packs in color negative or positive reversal films.
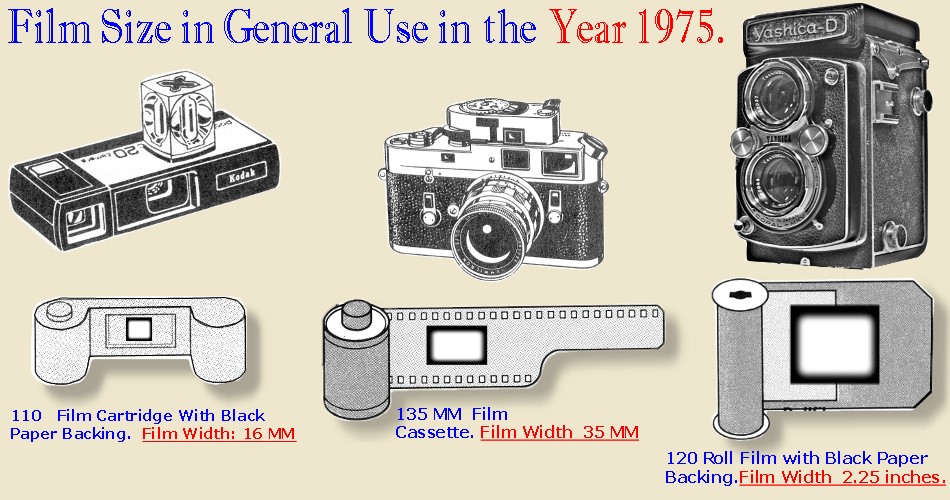
COLOR MATERIALS AFTER 1936
THAT REPLACED THE " AUTOCHROMES " OF 1907
since 1936 color couplers were added to 3 separate emulsions layers coated on a
single support. it could be processed in a single color developer. The same goes for all brands of color
emulsions on paper or celluloid films. the author of this work processed in 1975
color positive and negative material. all used a color developer called CD4 that you could buy from many different suppliers to make your own C41 process color developer. after development a potassium ferrycyanide bath was used to
bleach and eliminate the original panchromatic black and white emulsion leaving on the support only the color dyes
tha made up the color image.
 HOW IT WAS
MADE:
HOW IT WAS
MADE:
Color Material, Negative
and Positive, on Paper or Film:
STEPS For the manufacturing of color material:
1. 3 different black and white emulsions each differently spectrally
sensitized to red green and blue, are coated on top of each other on the same support, and after exposure:
2. in the developing stage, silver images are formed simultaneously along with
dye images.
3. Each of the black and white images developed are afterwards eliminated by
bleaching them, leaving only the corresponding color dye images on top of one another exactly where the original
silver monochrome images had been, Forming thus is formed the final color positive or
negative image.
4. this color process was used for positive or negative color photography on paper
or plastic Flms. there is no basic difference between a POSITIVE AND
NEGATIVE EMULSION
they are made exactly the same way, except the "positive"emulsion is
made with more contrast and less sensitivity than a "negative"
emulsion. in negative emulsions to gain more sensitivity to light, potassium iodide is added to the gelatin along
with the potassium bromide to heat a longer time without fog and get a larger silver grain.
a rapid negative emuilsion can be coated on paper to make positive prints, but contrast will be very low.
all color material is based in the starting sensitivity of monochrome (black and
white) gelatine silver bromide emulsions. The grain of color film was more forgiving, The sensation of color
made the grain less objectionable.

For the reasons explained above, 200 ASA silver grains on these could be
accepted, but never for enlargements over 8x.
1975, Highest Color
Film speeds to daylight:
Reversal positive transparencies: ASA 160 Color Negative Film: ASA 100 These were good emulsions, with High Resolution and Fine Grained. Quality 8x
enlargements could be made.The patented "T" giant grain technology of
the 1980s by which Kodak claimed to have improved its ultra speed color film
emulsions, (ASA 1000) even 25 years later, in the
year 2000, was yielding poor quality in their ultra
speed color films.
Speed Limit of Emulsion Quality
The ideal in the 1880s was to reach the sensitivity shown on the highest number
on warnerkers sensitometer, which was 25 degrees (ASA or ISO 8) that speed had
been reached and surpassed by the year 1900.
But there was a limit in quality in the race for emulsion sensitivity that started in
the year 1880. None of emulsion workers still alive in 1935 knew what
this limit would be. But it is known now, The limit was "37 degrees Warnerke " Equivalent to 2000 H
& D or 125 ASA or ISO.
EXPOSURE TABLE
for Front lit subjects under Full Sunlight at noon
Landscapes and portraits. All with a lense aperture at f 4.0
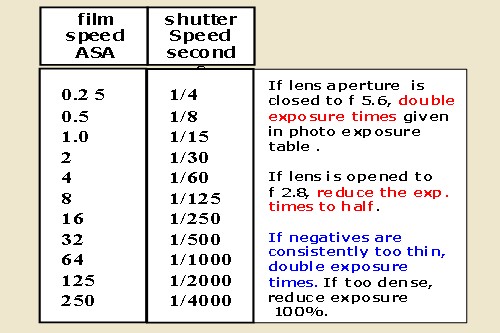
Notes:
for ASA 25 at f 4, expose 1/200 th of a sec.
For ASA 12 = 1/100 sec. ASA 6 =1/50 sec and so on.
saul bolaños, i, the author of this book write:
" I can assure you that no process known can produce high quality photographic images direct in
uneven surfaces like stones or concave ones like eggs, or convex ones like
spoons. " - it was to have no limits as an artist, that i became and expert in gelatin silver
emulsions. it is better and much faster than painting.

this is possible because for printing
the image made of light is projected
onto the surface which is made previously light sensitive with a silver emulsion, the projected image
conforms sharp and exactly to "any shape" of the object. after exposing the coated light sensitive
material, this is immersed in a liquid developer solution to make the image appear like magic.
Synopsis:
THE HIGHEST EVER REACHED GELATIN SILVER BROMIDE EMULSION SPEED WITH NO LOSS OF QUALITY WAS ASA or ISO: 125
1. for miniature film users
Ultra speed negative emulsions appearing after 1945
were of poor quality The Emulsion precipitation times were too long; although with tolerable low fog; - the
grains required to trap so much more light energy that unavoidably they were made to grow too large.
2. of enormous help was:
A. GELATIN no medium is better to protect and
impart sensitivity to silver salts.
B. sensitizing dyes for "
ortho " or panchromatization.
C. Lupo Cramers precipitation technique.
D. Sheppards Sulphur Sensitization.
E. Kolowskys Gold sensitization.
 Around
the year 2005
Around
the year 2005
threatened by Digital Photography The Gelatine silver bromide as a process for
the masses was being forced into retirement.
It had served the world well, better and longer than any other industrial process in history. 5 generations, More than 125 years But it will still live
a lot longer servicing x ray in radiography, the movie industry in
cinematography and last but never least, The artists and scientists, which after all, gave it birth.
Nothing or No One, Can stop Progress.
the Universe Governs Itself By the Laws of Change !
digital photography will be replaced also sooner or later.
END OF CHAPTER 1

above the author of this book taking a portrait.
only professional
photographer used large format cameras in 1975
the general population used small format 110 and 135 mm.
ONE OF MY BOOKS:
" HOW TO MAKE PICTURES WITH COFFEE"

TEXT MESSAGES
IN
COSTA RICA
6012 4695
![]()
CONTACT

COSTA RICA © 2025, saul bolaños
CAFEDESAUL@GMAIL.COM