CHAPTER 2


SAUL BOLAÑOS.
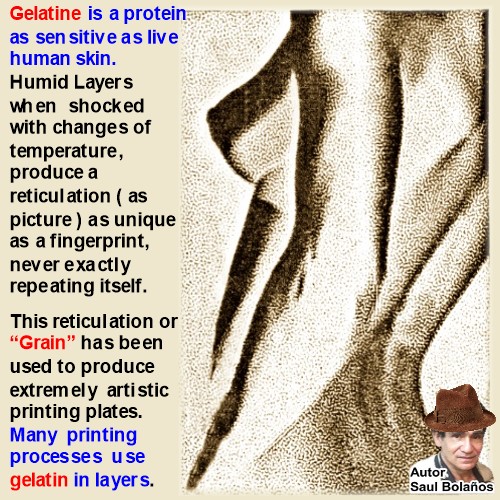
I have written this book to be useful Today and even more tomorrow when all experts on this art will have gone away.
It is a Valuable tool for Artists, Scientists and Students.
It is not about Theory, It is all about Practice with explicit Working Directions & illustrations of my own authorship.
On Some
Subjects I have given both “Text” and “illustrated “ Chapters as well,
This was done
Not to repeat myself, but for the sake of Clarity.
 SOME OF MY RESEARCH
SOME OF MY RESEARCH -Discovery of Yerba Mate as Reducing Agent.
- Invention of a stainless high energy Coffee Developer for Photographic Film
-and Paper.
- a printing process based on Light Sensitive oils derived from the coffee plant.
- coffee pigment prints
- Invention of a new process
via silver salts
to make Indestructible Photographs
which are not affected by fire, water or gases.
 -Also of the first Coffee Toning process for silver based prints or Silver Emulsions.
-Also of the first Coffee Toning process for silver based prints or Silver Emulsions.
-and of the first panchromatic LIght Sensitive Oils which were made to see the full spectrum of color light radiations as the human eye sees it .
-invention of a new type of silver emulsion stabilizers for high speed photographic films and paper.
-And of Full Color Edible Images As Disclosed in The United States Patent Office.
This was the first imaging process ever to produce full color half tone "Edible" photographic images on any solid food stuff such as
bread, cookies, cheese, etc.
or on Semi liquid foodstuffs like ice cream.
invention of 100 % edible coffee images - of 100 % cocoa edible images - of 100 % powdered milk
edible images - of 100 % black tea edible images - of 100 % yerba mate edible images. -ultra rapid silver chloride gelatine emulsions based on ultra active gelatin due to a process of gelatine purification.


Photo Grade Gelatin
Usually comes in Powder form or in pebbles but it may also come a in Leaf or Sheet form. It is made from calf hides and ears. Pig’s skin is used for the preparation of some gelatines. Large quantities are also made from bones; the calcium is eliminated to form Ossein. The hair and fat is removed by soaking in lime water for 2 months. The alkaline lime is washed off with dilute acids. Hydrochloric or sulphuric) The residues are washed and cooked to extract the gelatine. About 5 cooks are practiced. The gelatin solutions are condensed by heat until thick, cooled and dried to form slices or sheets. Only the first 3 extractions are used for photographic gelatin.
 The first extraction is done at 60c for 3 hours, the second extraction at 70c and the third at 80c German photo-grade gelatine manufacturers employ only the first extraction, it is said that its finer qualities are more reproducible.
The first extraction is done at 60c for 3 hours, the second extraction at 70c and the third at 80c German photo-grade gelatine manufacturers employ only the first extraction, it is said that its finer qualities are more reproducible.
The second extraction they use for food grade or edible gelatine. The last extractions may be used for manufacturing glue. Gelatine can also be made by acid process. Treated also for 2 months.
Acid gel differs from lime gel. Gelatine from animal hides contains Keratin and about 1% albumen. Both of these contain sulphur which is very important for high speed emulsions. Albumen is also very rich in anti foggants and emulsion ripening retarders. I use Food Grade GELITA Leaf Gelatine for all my ultra-high speed negative and positive slow emulsions. This is an inert type which I dose with Sulphur. Sulphur
Minute quantities or sulphur containing substances, for example in the form of thiosulphate present in 10 to 200 parts per millions can exert a great influence on the activity of the gelatin to produce high speed emulsions. Thiosulphate is probably produced by the degradation of the amino acid Cysteine during the liming process.
But sulphur bearing compounds are not all sensitizers,

Cysteine is a sulphur bearing amino acid and it is itself a powerful crystal growth restrainer and also reacts with aldehydes present in gelatines to give thiazolidines
which are in themselves powerful restrainers and anti foggants according to Steigmann. Some types of Aldehydes naturally present in gelatines can cause fog.
Oxidation
Photo Grade ( and even the best of “ Food Grade” gelatine are becoming more generally available now as “inert” types,
Gelatine is submitted to process of purification or oxidation which removes Sulphur compounds and all fogging agents to make it inert. All of these Gelatine variables will be dealt with in this Chapter.
 IMPORTANT
IMPORTANT
I have divided gelatine types in an arbitrary manner, but in an extremely practical way according to their behavior in emulsion Manufacture, which is after all what really matters. See my gelatine Table. Following are some important facts for workers who can not buy gelatin in very large amounts:
1. Most “Photo grade” type of gelatine sold today in small quantities come in powder form and is “oxidized” or an “inert” type of gelatine. This oxidation process removes its natural sulphur sensitizers. These gelatines are “Inactive” and require to be sulphur sensitize to furnish high speed high contrast emulsions. This type of gelatine in this paper is treated arbitrarily as “ TYPE IV” see below in " gelatine table" .
2. High quality “ Food grade” that is, “edible” gelatine come only in Leaf or Sheet form, Most types of these gelatines are good and now come also “oxidized” or “inert” and require as well to be dosed of Sulphur in the form of Thiosulphate to yield high speed high contrast emulsions. This “ Food Grade” gelatine in Leaf form is also a good “inactive” gelatine for emulsion making.
 3. “Food grade”
3. “Food grade”
gelatines in powder
form are usually “active”
they have to be avoided like the Plague they vary greatly from one batch to another, and yield for the most part, very high fog and lower contrast in emulsion making. This gelatine in this paper is treated as: “ TYPE X” gelatines. In all cases:
A gelatine has to be tested by actually making an emulsion.
For Testing use my Emulsion formula #1 ( which see).
Long Liming Gelatines made From Bones... are naturally less “active” and more inert than the other types. Gelatines with lower amounts of “restrainers” are produced by longer washing times, while gelatines produced with shorter washing times consequently keep their natural restrainer; these are less “active” simply because they have been left with higher amounts of natural restrainers. Hide gelatins usually contains more sensitizers and are more “active” than those made from ossein due to treatment and method of removing the hairs from the skins.
Gelatine produced from hide or bones of small animals like rabbits are very pure, these gelatines have little or no sulphur and is rich in fog inhibitors. Food grade gelatine made from hides or bones from cows grazing in volcanic regions are very active to the point that they may produce emulsion of extreme high speed but with great amounts of fog. These are rich in sulphur but without enough restrainers. Nevertheless these inconvenient properties, an experienced photo grade gelatin manufacturer could substantially improve its photographic properties.
Photographic gelatin manufacturers have some control over how much of sensitizer or restrainer is intentionally left on their gelatines. Their methods are of course trade secrets but despite the fact that some of them are brilliant emulsion chemists, they are not particularly enlightened, but when it comes to the chemistry of gelatine no body is. It was Sheppard who first discovered that much of the light sensitivity of photographic gelatine was imparted by minute traces of sulphur. Working for and at the Kodak Research Lab, He also discovered at least some types of restrainers.

PH of Gelatin
Isoelectric point of substances is the ph value at which is electrically neutral, so under the force of a magnetic field it moves neither to the negative or positive pole. Gelatine is amphoteric; it can act either as a base or as an acid. Lime processed gelatin's isoelectric point is PH 4.7 – 5.3 Acid processed: from PH 7 to PH 9. ( Ph 7 is neutral, below 7 it is acid and above 7 “ Basic” or alkaline.
PH of EMULSIONS: Full Ammoniacal emulsions after washing have a PH of 11,
Neutral Emulsion (without Ammonia) have a PH of 6.0 -GOLD SENSITIZATION: PH of Emulsion must be below PH 8, ( Optimum: 6.2 - 6.5 ) At the start of Second Ripening; PH must be adjusted, it may be lowered by addition of 10 % Sulphuric Acid or Sodium Bisulphite. Or raised by addition of 10% Sodium Hydroxide or Sodium Carbonate. For raising The PH to 6.2 of a neutral emulsion for example, to every 200 ml of emulsion add 1 ml of a 10 % Sodium Carbonate. 2ml of the carbonate will raise it to about PH 6.4
 Hardness of gelatine
Hardness of gelatine
the hardness of gelatin is measured in degrees bloom. The hardest gelatin being about 257 bloom and the softest 53 degrees bloom. Hard, very mechanically resistant gelatine with a higher fusion point and considerably stronger viscosity when melted by heat in liquid form is obtained when the animal skins or bones are treated first with acid for about 6 days at room temperature and then with lime for about a day. This combined acid /alkaline treatment produces gelatine of 257 degrees bloom which is about twice as hard as gelatins produced by the normal either acid or alkaline liming process alone.
Soft Gelatine
The softest gelatin ( 53 bloom) is obtained by a “neutral” process which is neither acid nor alkaline. Medium Hard Gelatins of 149 bloom are yielded by a short all strictly acid treatment. Prolonging the acid treatment time produces even softer gelatines of 125 bloom. Medium hard gelatines of 152 to 157 bloom can also be fashioned by the alkaline liming process alone, without any acid treatment.
Gelatin Hardness in Emulsion Making average normal gelatin hardness for emulsion making is about 150 bloom. Much softer gelatins can of course be used. By mixing a 257 bloom hard gelatin with equal parts of 53 bloom soft gelatin, a medium hard gelatin of 155 bloom is the result. Gelatine hardness should not be a primary concern in formulation of emulsions for beginners, Much More More Important is its activity. (see below). Generally speaking, the harder the gelatin the less amount it can be used in relation to the silver content because it can stand more tear and wear during wet processing than softer gelatines if used in same amount.
But the emulsion can be dosed with a higher amount of softer gelatin to attain similar results. The proportions for high speed emulsions for negative work, where higher silver content is desired to obtain more density and more sensitivity would be about:
Edible “Food grade” type of gelatine in Leaf form is generally a “soft” to “medium soft” type and can be very well used in Emulsion Making. “Food grade” gelatine in powder form is usually a hard type of gelatine, usually above 200 Bloom.

How Much Gelatine in Proportion to Silver
Much more gelatin can be used for positives on paper than the proportion to the silver given above, in such case a ratio of 4 parts gelatin to every 1 part of silver nitrate is about the limit for high quality work. Too much gelatin in proportion to silver content is to be avoided because:
It causes the whites to go yellow during development which by the way takes place at a slower rate, it also tends to fix fixer stains, it dries much slower and image will not have its maximum sharpness. In some types of emulsion where the silver grain may have grown to be large and translucent and does not have great covering power; higher gelatine content in such case: -may drop maximum blacks lowering contrast and at times it may lead to speed loss.
Hardeners for gelatin:
-Formol, -Chrome Alum, -Potassium Alum
Hard gelatines adhere better to bare glass plates, that is, glass which has not being pre-coated with a subbing layer to anchor the emulsion. But softer gelatines will adhere equally well on subbed glass. If over hardened with common gelatin hardeners like chrome alum, potassium alum or formalin, introduced in the emulsion for the purpose, hard gelatines as well as soft gelatines may lower contrast or even reduce the speed of the plate due to the over hardened gelatin repelling the developer solution during processing. Hardening agents quantum is critical for reasons mentioned above.
The author of these lines has vast experience on the quantum of hardening agents, and these are given in several places in this book. Maximum hardening of gelatine dosed with hardeners, takes place usually after 7 days after coat has dried. But, even within 48 hours, the presence of hardeners is made evident, as the emulsion layer is seen to withstand much longer wet times without damage.
 Adequate hardener
Adequate hardener
Quanta is given below.
To Harden Gelatine in emulsions add the quantity of one of the solution given below per every 10 grams of gelatine ( dry weight )
For example quantity of either formol or chrome alum may be added to every 100 ml of an emulsion containing 10 % gelatine. Emulsion will not be “over” hardened with dosage given.
Note: Add just before coating, with dosages above emulsion if not coated all in one single session, may be liquefied again. Do not keep emulsion dosed with formalin in liquid form under refrigeration for a long time, it may polymerize making it impossible to liquefy again. For an extra hardening effect,
for example for coatings on glass to be used in tropical climate, both hardeners the alum as well as the formol given above may be added together to every 10 g of gelatine ( dry weight) in emulsion.
A maximum of 1 ml solution of 10 % formalin can be dosed per each 10 g gel ( without the chrome alum) with no adverse effects, providing no cupper salts have been added to emulsion.
the larger dosage of formalin (also known as formaldehyde) tends to overharden if emulsion is made too alkaline. Alternatively,
instead of introducing the hardening agent in the emulsion itself, the hardener may be added to the fixing bath. This has the advantage that the full emulsion speed and contrast is retained as the soft unhardened gelatin layer absorbs the developer readily. For this last purpose wetting agents and/or plasticizers and hardener may also be included and introduced together in the emulsion, a common
 Emulsion Hardener and Plasticizer
Emulsion Hardener and Plasticizer
plasticizer like glycerin makes the coated layer more pliable and prevents it from over drying on long storage.
For example, a good hardener and plasticizer may be introduced as in solution A. (see picture)
which keeps indefinitely, Use same proportions for different quantities of emulsion of which gelatine content is of course always known.
Gelatin Chemistry
For chemical or photographic properties the situation with gelatin is overwhelmingly intricate and a lot more difficult if not impossible to predict. Gelatin is an extremely complex protein;
its molecular weight has not even been determined yet, it has been estimated to be about 30.000 to 100.000. It has a legion of organic substances which can influence the properties of an emulsion. These Compounds can modify contrast, raise or lower sensitivity, inhibit or promote fog. Broadly speaking "restrainers" in gelatin can retard fog and in combination with "contrast promoters" raise contrast but also hold back sensitivity.
 Gelatine Sensitizers
Gelatine Sensitizers
"Sensitizers" in the gelatin can promote sensitivity but may lower contrast and accelerate the emergence of fog. There are also 'fog inhibitors" which defend the silver crystal from being reduced to metallic silver during precipitation. Gelatines have also crystal "protectors" which surround the silver grain protecting it. Without this "protectors" the emulsion without exposure to light would be fogged by the developer. Other ingredients naturally present in gelatines help retain the latent image.
The exact constitution of each type of gelatine varies according to the source of raw materials and its method of treatment. Gelatins are not all alike. Some are excellent for emulsion making; others are extremely poor and others are just average. Consequently, Very Broadly Speaking, gelatins for the purpose of simplicity may be divided in only 3 types: those of medium activity, Active and inactive.
Medium active gelatins would not produce emulsion of high speeds but would cause low fog and medium contrast. Active gelatins can yield extreme speeds which are a challenge to stabilize assuming fog can be controlled by the expertise of the emulsion master. Inactive gelatines may be rich in restrainers that while imparting low speed to the emulsion can render high contrast and inhibit fog. At present most gelatines are being oxidized, this makes them inactive, and must be dosed with sulphur to obtain high speed and density. “ Inactive” in this sense is a synonym of “inert”. (see below)

all of the gelatine used to formulate all my photographic emulsion given in this book are "Gelitas" soft leaf gelatine tasteless and odorless. it is an excellente inert type of gelatine can be dosed with sulphur (thiosulphate) to yield high contrast and extreme speed negative silver emusions as well as very clean positive emulsions for paper and glass.
The "ideal" gelatin would be one that can make a high speed emulsion with high contrast and low fog. The ideal gelatin does not exist but at my Research Labs we know “it can be be crafted”. It has been said that the "art" of emulsion making is in great part the skill of choosing the gelatins. Easier said than done. But that is certainly the case. For example; -Precipitation can be carried out in the presence of an inactive gelatin. At end of precipitation a medium active gel may be added. After washing and melting, for chemical or second ripening a very active gelatin may be added in amounts comprising about 10 - 50% (depending on activity) of the total bulk of all gelatins employed.
When, what type, how much? Answers to those questions were well guarded secrets of the trade. On the other hand one does not have to be a master to make a respectable emulsion with a normal type of gelatin or even with a food grade one. However it may take a few trials to correct errors but careful notes and attention to details, besides a good formula, are the principal ingredients. All formulas in this book I worked out myself. They are Very Good and only use one type of gelatine: GELITAS TASTELESS AND COLORLESS LEAF GELATINE. Emulsion Formula for Testing Gelatines
An excellent emulsion formula and procedure is given in this book for the testing of gelatines and for making a good emulsion with most types, even “food grade“ edible gelatines. ( see my # 1 Ammonia emulsion).
 Sulphur
Sulphur
Gelatines very rich in sensitizers, the so called “active” contain from 20 – 30 grams of ammonium hyposulfite or thiosulphate for every 100 kilos of dry gelatine. Gelatins of medium activity, also the Active type, as well as inactive gelatins are articles of commerce. Photo-grade commercial gelatines of the inactive type may contain from 1 to 4 grams of thiosulphate for every 100 kilos of gelatine (dry weight). The thiosulphate is also known as “hypo’ or sodium hyposulfite which is the ordinary photographic fixer. The sulphur, in the form of thiosulphate is introduced by the gelatine manufacturers.
Most German gelatin manufacturers introduced the sulphur on the average of 1 gram of ammonium hyposulfite per every 10 kilograms of gelatine to make it an active type. Sulphur in many forms is bonded to a great variety of organic compounds all native of and naturally present in gelatin. It is a ripening accelerator, it is compulsory for high speed emulsions.
Retarders and Antifoggants:
To slow the ripening on the other hand, ripening retarders like Cystine, another natural constituent of the gelatine, may be introduced at the rate of 1 gram per every 10 kilos of dry gelatine. Up to a limit, Gelatines can be enriched with desirable components or disposed of its undesirable ones. Chondrine for example, also naturally present in gelatins, produces intense fog in emulsions. Albumen , retards fog.
Many other compounds beyond the scope of this book , play a significant roles in emulsion quality. Inert Gelatines
“Inert” types of gelatins are in theory gelatins which have been purposely disposed of sensitizers and restrainers. At least in enough amounts to the point where they are rendered incapable of entering or surrounding the silver bromide molecule and inhibiting it from coupling with the gold, or sulphur compounds, which are added intentionally at specific amounts at the stage of emulsion making, under controlled conditions. All of these for the purpose of extreme speed with fineness of grain in emulsion manufacturing.
 Completely Chemically Inert
Completely Chemically Inert
the author of these lines worked out a process to make gelatine “totally inert” But on making high speed negative emulsions, they invariably produced fog
simply because the chemical treatment stripped the gelatin of all its natural constituent, the bad ones as well as the good ones. Washed away were their natural restrainers, their fog inhibitors and grain protectors. These gelatines made “inert“ by chemical means, require to be synthetically dosed with some type of restrainers to inhibit fog and some type of sensitizer like sulphur to produce great light sensitivity.
Unknown to the author, P. Askenazy ( 1926 ) had already found this out, He removed by absorption methods, all substances out of the gelatine and received very high ripening unstable gelatines. But after restituting or re-adding the isolated fog inhibiting substances subtracted in the first place, ( like albumen and cystin)
the original stable state of the gelatine was reestablished. Bottom-line: Truly “inert” gelatines are useless. But the literature refers to “inert” gelatines, to gelatines which are “inactive” in other words -without sulphur, and fogging and speed sensitizing compounds, but these improperly so called “inert’ gelatines however, are left with their natural retarders in enough amounts to defend them against fog and to produce stable predictable emulsions.
This point has never been clarified in the literature when referring to “Inert” gelatine. This term is generally employed and is in fact misleading

Today most gelatine are being made inert by “oxidation” processes, which do not strip it of its natural fog inhibitors, but remove sulphur and fogging agents as aldehydes. These gelatines are stable and appropriate for emulsion making. They appear in commerce as “Photo Grade”, but only a test can show for sure how active or inactive they are.
There are many good types of gelatine for emulsion making in the market. The time has to be taken to find them. Even some types (leaf form) high quality “Food Grade” gelatines have given the author excellent fast, clean, contrasty negative emulsions. Gelatines do not have to be made “inert” chemically but for some purposes it may be more convenient. to strip it from all its natural constituents. But as said earlier, chemical purification of the gelatin may make very poor gelatine good.
But in the other hand since the treatment strips it of all its bad as well as its good properties, it can make otherwise good gelatine to behave very poorly.
Inert gelatins required for extreme emulsion speed in gold sensitization can be prepared by washing the gelatin with acids, neutralizing the acid with alkalis, washing and drying. To make a gelatine so called “inert” according to steigmann we particularize, noting that this procedure is not at all necessary for good Emulsion making, but it is given here for the purpose of education only
To make gelatin Inert
10 grams of the gelatin, powdered or
in leaf form is soaked for 24 hours at
a temperature of 5 - 10 c in solution of:
citric acid 2 grams
water distilled 300 ml
After 24 hours the excess acid water, is drained off. (Unwanted substances pass into the citric acid) The swelled gelatin is melted at 40-45c, the PH raise from 2.5 to about 4.5 with sodium hydroxide (corrosive) . The gelatin solution is now set in ice water. Shredded in noodles just like it is done for emulsion washing, ( which see) and washed by soaking 6 times in:
Distilled water 300 ml
temperature 5-10 c  About every 6o minutes the water is changed. Washing will take about 6 hours and it will take about (6x300ml) =1800 ml of water. After washing the gelatin is gathered, strained and dried. This can be done by extending it over a plastic mesh with large openings so it can be well ventilated. Drying can be accelerated with currents of cold air. The above proportions should kept even if more 10 grams of gelatin are to be purified. This treatment gets rid of ripening retarders and sensitizers but not the aldehydes, but for all practical purposes it is generally good enough In Sulphur Sensitization,
About every 6o minutes the water is changed. Washing will take about 6 hours and it will take about (6x300ml) =1800 ml of water. After washing the gelatin is gathered, strained and dried. This can be done by extending it over a plastic mesh with large openings so it can be well ventilated. Drying can be accelerated with currents of cold air. The above proportions should kept even if more 10 grams of gelatin are to be purified. This treatment gets rid of ripening retarders and sensitizers but not the aldehydes, but for all practical purposes it is generally good enough In Sulphur Sensitization,
it is customary to precipitate the emulsion with an inert gelatin and to first ripen under a low PH, then after washing, an active type of gelatin is added or instead the sulphur compound itself is introduced, the PH is then raised and the emulsion is ripened until the first traces of fog begin to appear. For many decades a legion of methods have been devised to chemically test a gelatin for the presence of reducers, sensitizers, sulphur, restrainers, aldehydes, foggants, etc. but the emulsion properties of a gelatin can never really be evaluated until an emulsion is actually made with it.
Constant Quality from Batch to Batch
The best gelatines for emulsion making are of course the photo-grade types. Mainly because they have a constant high quality that is maintained from batch to batch, This is of Capital Importance to reproduce consistently any given emulsion. Food grade gelatins of reputable makers, although still more unpredictable, can be conditioned to produce respectable good emulsions, Providing the factory issues a constant quality from batch to batch.
 PRACTICAL SULPHUR
PRACTICAL SULPHUR
SENSITIZATION
I usually work with 2 types of Food grade Gelatin in sheet form, and with one type of food grade gelatin in powder form. These gelatins are all from different manufacturers.
All conditions being equal, the powder gelatin is extremely active, with tendency to fog, it produces with all emulsion formulas, about 3 times more speed than the sheet gelatins. This is not really a good gelatin it has not been “oxidized” and it is loaded with natural fogging agent.
One of the food grade gelatins in sheet or Leaf form mentioned above, provides invariably good clean emulsions with superb dense blacks, excellent contrast with ammonia emulsions for bromide paper. This is a normal gelatin of medium activity...capable also of producing high speed for negative work under special somewhat too complex for beginners; precipitation techniques.
This is good gelatine for all around positive and negative work The second type of food Grade gelatine in Leaf Form is very inactive, (gelitas leaf gelatine) it is “oxidized” Regardless of the silver bromide formula, this gelatine after precipitation and first ripening and after washing in the usual manner,On melting for the second ripening, it does not matter how long the emulsion is heated, it never produces strong blacks, its low speed remains constant and never produces fog, the whites are clean even after 24 hours at 55c.
(Heated in enclosed container to avoid condensation by loss of water) It is a good gel to sulphur sensitize for “High Speed” and "contrast" positive formulas. Gelitas leaf gelatins are sold worldwide but are packed by many local companies in many countries under of hundreds of different brands.
 This Gelitas gelatine never yields strong blacks. The darkest shadows are only medium to pale grays. If coated on paper. contrast is very low, about grade 1.0 a useless gelatin for bromide paper, and too insensitive for negative work. I thought I would best eat it. It is a beautiful sheet, transparent, of unusually pale yellow color and almost odorless. But then, I though of introducing some sulphur to it during emulsion making. When it is sensitized with sulphur, the grays come alive turning into superb blacks with great contrast and good speed in proportion to the emulsion formula.
This Gelitas gelatine never yields strong blacks. The darkest shadows are only medium to pale grays. If coated on paper. contrast is very low, about grade 1.0 a useless gelatin for bromide paper, and too insensitive for negative work. I thought I would best eat it. It is a beautiful sheet, transparent, of unusually pale yellow color and almost odorless. But then, I though of introducing some sulphur to it during emulsion making. When it is sensitized with sulphur, the grays come alive turning into superb blacks with great contrast and good speed in proportion to the emulsion formula.
The exact amount of sulphur took several tests to define. With Sulphur added in excess the emulsion gains more speed than usual but the whites go gray with heavy fog. This fog may be gray; of all shades of yellow or even orange. Excess sulphur tends to fix developer stains during processing. It does not work clean. With amounts below the optimum in sulphur, speed is not maximum, the contrast is medium without sparkling blacks, and the whites remain clean with No fog. With optimum amounts of sulphur : speed is maximum, blacks are dense and brilliant and the whites pure. Today this Food Grade Gelatine in Leaf Form is my favorite gelatine and was used for all the emulsion formulas, negative and positive, given in this book .

I use “pentahydrate” hypo to introduce the Sulphur,
and for the only reason I use this salt ( and not others) is because I have great amounts in stock and it is very stable. If the anhydrous salt is used, a lot less than the amount given below has to be used. The prepared solution has a life of no more 3-6 months under refrigeration 5-10c. If stale sulphur solution is used, contrast will be below optimum, and speed loss may be from 25-50 % Blacks will not be dense. (Micro-organism in the solution develops with time and feed on the sulphur rubbing it from its strength)
How To Sulphur Sensitize and Test. Note: if gelatine is of a good medium activity and produces good blacks and good contrast sulphur sensitization may result in a higher speed emulsion but in some cases with a degree of fog that may be intolerable. After washing at the beginning of Second Ripening add:
 As said earlier exact dosage of sulphur for the type of gelatin has to be pre-tested.
As said earlier exact dosage of sulphur for the type of gelatin has to be pre-tested.
For example:
an emulsion made with 10 grams silver nitrate, after washing and melting is divided into 5 equal lots. Each lot will be known to have 2 grams of silver (seen as nitrate)
For the purpose of testing, one of the lots without the sulphur is given a second ripening of say 1.5 hours at 50c. This first batch will serve as “control” providing all conditions (minus the sulphur ) are duplicated in the second lot of the emulsion. This second lot now will be dosed with the sulphur and also submitted to a second ripening time of 1.5 h / 50c.
By comparing the first with the results of the second, the effects of sulphur sensitization will be appraised. With this last “ Food Grade” inert type gelatine mentioned, I have attained, with sulphur sensitization alone and very complicated precipitation techniques (without gold or spectral dyes) emulsion speeds of ISO 64, !!! excellent for negative work when coated on glass plates.
If spectral dyes were added to make this emulsion “Ortho” sensitivity to daylight would substantially increase to a speed of about 100 which is the maximum speed ever attained consistent with excellent resolution, high contrast and fine grain for high quality photographic negative work. And all with 1 single emulsion type coated on the plate.
HOW TO PREPARE SODIUM THIOSULPHATE.
USE AS PHOTOGRAPHIC FIXER AND GELATIN SENSITIZER
it is not dangerous but prepare this in the open air. but the odor is not pleasant and it will fog photographic material. avoid breathing the boiling water vapors. in a 4 liters stainless steel pot mix:
water.......................................................... 1 liter
yellow powdered ordinary sulphur .........25 grams
sodium sulphite .....................................165 grams
Heat at boiling point for 2 hours with lid on pot so as not to loose too much original volume of water. Violent boiling will not speed up dissolution of the sulphur. at the end of two hours evaporate solution to give final volume of 500 ml. cool and filter off any remaining un dissolved sulphur. the filtered odorless solution is about 30% strength in sodium thiosulphate. it will fix any silver based film ( even X ray film) in about 1 minute. it will keep (with out acid indefinitely in a cool dark place)


CAPACITY: diluted 1:1 water (15%) each 40 ml will fix an area equivalent to 5x7 inches of silver based photographic material in 5 minutes. diluted 1:2 with water (10% strength) 60 ml will fix it in 10 minutes. ( photo paper requires only 5 min) as soon as those given areas have been fixed discard the fixing bath. it may be acidulated with about 1/5 its volume of white vinegar to avoid stain. it is also known as sodium hyposulfite.
DISPOSAL: can be safely flushed down the sink with plenty of water. a plain sodium thiosulphate solution has a extremely low toxicity (if any) but do not drink it. Protective gloves need not normally be worn to handle this compound or its solutions. the working fixing solution may be used without adding any acid or, to prolong its life and keep stain to a minimum, acidulate by mixing with equal parts of 5% boric acid solution, which is also non toxic.
FOR EMULSION SENSITIZATION: add 10 ml of the 30% solution to 3 liters of water. Strength is 1:1000. Dosage: 1 ml ( of 1:1000 strength) per every 1 gram of silver nitrate in the emulsion. At the same time add: sodium carbonate anhydrous 10 grams dissolved in 100 ml of water: of these use O.5 ml per every 20 ml of liquid emulsion. Make tests and keep notes.

HOW TO MAKE SODIUM CARBONATE AT HOME Buy sodium bicarbonate (Baking soda) in any store or pharmacy. It is used as an anti acid for heart burns. Place about 50 grams ( or 1/2 cup) of the powder in a steel pan and heated it for 10-15 minutes strongly directly over a household burner set on "high". the bicarbonate rapidly decomposes into the "carbonate" at very low heat (200c) further heating will make it Anhydrous.
Do not worry about overheating your heating element will never reach the 1000c necessary to convert it into the oxide. to check when it becomes anhydrous by loosing all its water; put on the lid on the pot for a few seconds, when it keeps dry and shows no water vapor i is dried.
After heating the bi-carbonate should loose about 40% of its weight and its solubility increased from 5 to 20%. Pack the dry carbonate and do not let it pick up moisture from the air. dissolve 20 grams of it in 100 ml of cold water to make about a saturated solution. keeps forever.
TEXT MESSAGES
IN
COSTA RICA
6012 4695
![]()
CONTACT

COSTA RICA © 2025, saul bolaños
CAFEDESAUL@GMAIL.COM