CHAPTER 3
CHEMISTRY OF SILVER BROMIDE GELATINE EMULSIONS
chemistry of silver halide photographic emulsion is in this page from my book: chapter 3. which recounts the chemistry of silver bromide and silver halide gelatine emulsions positive and negative. liquid light sensitive silver emulsion chemistry is a a good chapter in saul bolaño's PHOTOGRAPHIC SILVER EMULSIONS EBOOK.
EMULSION
Silver bromide is formed by double decomposition or chemical interchange between an alkaline bromide, usually potassium or ammonium bromide and silver nitrate. If aqueous solutions of these two salts were mixed in a haphazard fashion there would certainly be obtained a coarse, granular form of silver bromide which would at once sink to the bottom of the vessel, and there might be an excess of either Silver Nitrate or bromide.
To prevent the immediate deposition to the bottom of the mixing vessel of the bromide, And to obtain a fine grain, a vehicle gelatin is added to the alkaline bromide solution, and the fineness of grain largely depends upon the proportion of gelatin used.
If too much gelatin, or too hard a kind, be used during mixing it is difficult to obtain high speed, as the gelatin acts as a mechanical restrainer; on the other hand, if too little is used, a coarse granular deposit is formed, and the emulsion tends to fog and thinness.When an alkaline bromide and silver nitrate are mixed together they combine in definite proportions according to their Molecular weights.
The molecular or combining- weight of Potassium Bromide is
119,
and that of Silver Nitrate is 170.
If these quantities were-weighed out exactly, whether in grains, ounces, pounds, or tons, or grams or kilogram's:
exactly 188 parts of Silver Bromide would be formed,
and there would be found in the water neither Silver Nitrate nor Potassium Bromide, there would be no excess of either, the Silver or the Bromide. but the
slightest error in weighing might give an excess of Silver Nitrate, which would be fatal to the emulsion in alkaline
development. ( it would fog) It is customary, therefore, to use an excess of Bromide or other salt in all emulsions
intended for development;

this excess varies in most formulas, and is governed by the process used, the method of precipitation, the presence of
other chemical reactants, the quality or activity of the gelatin and the speed required. Some gelatins will give
perfectly clean emulsions with a much smaller excess than others. ( see chapter on
Gelatine) Then, as one of the prime uses of the excess of Bromide is to keep
the emulsion free from fog, a reasonable excess is useful on this account, and
increase may make an otherwise foggy-working formula satisfactory.
An increased excess of bromide tends to give faster emulsions for
negative work, but it has at the same time a tendency to produce thinness in the high lights in the negative plate,
that is density on the glass corresponding to the brightest parts of the scene, is wanting. In other words great
excesses of free bromide produce high speed, but with too low of a contrast.

Excess of Soluble Halide
Alkaline Metallic Elements like Potassium and
Sodium when reacted with elements like Chlorine, Bromine, and Iodine, produce the corresponding salts as follows:
Chlorides from Chlorine, Bromides from Bromine, Iodides from Iodine. All of these salts are called “ Halides” and they
are “ Soluble” in water. If any of these soluble halides, is reacted with Silver Nitrate, they produce “ insoluble”
Silver salts or insoluble Silver halides as follows:
-Silver Chloride from reacting soluble Sodium
Chloride (ordinary Kitchen salt ) with Silver Nitrate.
-Silver Bromide from reacting soluble Potassium or
Ammonium bromide with Silver Nitrate.
-Silver Iodide from reacting any soluble iodide
like potassium, sodium or ammonium iodide with Silver Nitrate.
A lot more than 10 % of potassium ( or ammonium ) bromide can be used to accelerate ripening and
yield higher speeds in negative emulsion making but it will also lower contrast considerably for positive work.
That is a fact firmly established and confirmed by the author. When first mixed the emulsion is very slow, no matter
what formula is used, and would be quite unsuitable for anything but for slow positive plates. It is therefore
subjected to a “Ripening” process, either by continued application of heat or
the use of ammonia. Exactly what occurs during ripening is a matter of doubt, but it is generally Assumed that the silver bromide grain increases in size and that this increase is accompanied by greater
sensitiveness to light; the change is probably more of a physical than chemical nature. The chemical interchange
between the silver nitrate and bromide, is represented by the following equation which may be said to be the chemist's
shorthand method of explaining what occurs:

The figures given are the molecular or combining weight, and, as has already been explained, 170 parts of Silver Nitrate combine with 119 of Potassium Bromide to form 188 parts of Silver Bromide and 101 parts
of Potassium Nitrate.
Alkaline Nitrate ( Potassium Nitrate ) thus Formed must be got rid of , and this
is the purpose of the washing, which also removes the excess of alkaline Bromide and the ammonia, if this latter has been used For Ripening.
Were these salts not washed out they would crystallize out on the plate during the process of drying after coating, and
either prevent the access of light, or give rise to -crystalline markings which would show in the negative glass plate,
or celluloid film.. If the unwashed emulsion was coated, on the other hand, on absorbent substrates like paper, for
example, then these soluble salts would eventually sink into the porous material.
POTASSIUM BROMIDE
Ratio For Emulsion Making. A normal ratio is 100 of Silver Nitrate to 80 of Potassium
Bromide, though it will be seen that the ratios given in some formulas vary from this in some cases. To saturate 10
grams of Silver Nitrate, 7 grams of Potassium Bromide ( KBR ) are required, but in general practice 8 grams of KBR are
used, which is about 10 % excess over the Silver Nitrate.
AMMONIUM BROMIDE
Ratio For Emulsion Making. If this salt is used instead of the Potassium salt to make an emulsion, In such case 5.76
grams are required to saturate each 10 grams of Silver Nitrate. But to leave the
Ammonium Bromide in at least a 10 % excess, ( to avoid fog ) about 6.5 grams of the
Ammonium Bromide would have to be used.
If Ammonium Bromide is used instead of KBR, First Ripening Time may have to be shortened about 25 % ( in Rapid Negative emulsion
making only ) The little ammonia set free from this salt speeds ripening and may
yield higher contrast in some cases depending on precipitation technique.
 Emulsion Making
Emulsion Making
Equivalent Quantities of Different Halogen Salts
Required to Saturate 100 Gms of
Silver Nitrate:
Ammonium Chloride ......................................... 31.5 gms
Sodium Chloride (Common Kitchen Salt)... .. 34.4 “
Potassium Chloride........................................... 43.9 “
Lithium Chloride................................................ 25.0 “
Calcium Chloride .............................................. 32.6 “
Calcium Chloride ( crystals)........................... 64.4 “
Cadmium Chloride.............................................53.8 “
Zinc Chloride .....................................................40.0 “
Strontium Chloride ( crystals) ........................78.3 “
Magnesium Chloride (crystals ) .....................59.7 “
Ammonium Bromide ........................................57.6 “
Sodium Bromide...................................... .........60.6 “
Potassium Bromide.................................. .......70.0 “
Cadmium Bromide................................... ........80.0 “
Lithium Bromide ..................................... ........51.1 “
Zinc Bromide......................................... ...........66.2 “
Ammonium Iodide................................... ........ 85.3 “
Potassium Iodide..................................... ........97.1 “
Sodium Iodide ...................................... ...........88.2 “
Lithium Iodide.................................................. 78.8 “
Cadmium Iodide.............................................. 107.6 “
Zinc Iodide........................................................ 93.5 “
How to use Table
As said earlier, to avoid fog in alkaline development, the halogen salts must be in
excess over the Silver Salt. Normal excess is 10 %, Let us suppose, we are
making a pure silver bromide emulsion with dry weights: gelatin: 40 g, and Silver Nitrate:10 g,
we want to use Ammonium Bromide to react it. We do this math: 5.76 g ammonium bromide required to saturate 10 g Silver
Nitrate. To leave a 10% excess of it: 5.76 x 1.10 = 6.33 grams is the result of which would be used to leave an excess
of 10 %, If we want to use 50 % halogen excess ( to gain more speed ) then we would use this math: 5.76 x 1.50= 8.64
gms would have to be used in this case. which could be rounded to 8.75 g.
Same math is used for any of the other halogen salts listed above. Using for each salt its corresponding weight to
saturate 100 gms ( or 10 grams) of Silver Nitrate.
Notes: 6 factors (but not the only ones) Influencing ripening rates
and contrast are: 1. The greater the amount of halide in excess over the Silver, the
more rapidly the crystal grows, That is; ripening occurs much faster. 2. The higher
the concentration of Precipitants the more rapid the ripening rate, 10 % pot bromide, added to 10 % Silver Nitrate ( in
the presence of 1.5 - 5 % gelatin) Ripen much more faster ( with larger grain/more speed/ less contrast) than same
precipitants concentrated at 5 % Independent of factors above; 3. the higher the
conc., of Pot Iodide the longer first and second ripening time can be prolonged without fog. 4. Mode of precipitation. 5. Quantity of gel during
precipitation. 6. Ph of emulsion. All Those variables work independent of each other
And the list goes on.
Chemical hazards
THE FINISHED EMULSION IS COMPLETLY NON TOXIC, but before
it is made the handling of some of the salts have its hazards. Potassium bromide, & Potassium Iodide, are not
particularly toxic.
 Silver Nitrate and Ammonia
Silver Nitrate and Ammonia
are the two chemicals that are hazardous & must be treated very carefully . Specially the Ammonium Hydroxide which
has a pungent smell and is very corrosive.
CHROME ALUM:
and SALICYLIC ACID:
Avoid contact with the powders when weighting it out, do not breath dust. The salicylic acid in pure form may irritate
the skin. Once diluted in the emulsion the dosage is so small of each of these that there are both harmless.
SILVER NITRATE comes as a white powder. keep away from light. Capped inside
bottles or packages in dry form it keeps indefinitely. On contact with organic matter it may darken under light but it
still can be used. Solutions keep for many weeks in distilled water. Do not dissolve silver nitrate in tap water, it
reacts with magnesium and calcium salts forming insoluble salts that make solution milky. HAZARD very toxic, may be fatal if swallowed. it may irritate the skin. Its colorless solutions
stain clothes and skin a brown or black color, stains appear only after exposure to light, on the skin they persists
for about 2-3 weeks and disappear.
protect from contact with eyes
(it can blind you) and handle
with gloves. Rinse any objects or dishes that contact it in solutions or dry
form. Once mixed with the gelatine and salts solution, silver nitrate transforms into harmless non toxic silver halide
suspended in gelatine.

AMMONIA (ammonium hydroxide)
for emulsion making use the strongest available: 25 - 35 % strength A volatile, pungent gas, which for
photographic and many other purposes is used in the form of a watery solution (NHSOH); The strongest solution, and that
mostly used, is of .880 sp. g., contains 35 per cent, of the gas NH3, and is commonly known as " ammonia
".880 " or " liq. ammon. fort. "Liquid ammonia" is the
incorrect, popular form of the term " liquor ammonias." A weaker liquor, kept by most chemists, one-third the
strength of the .880 solution, is rarely used in photography. Ammonia has many uses in photography, the ammoniae should
be kept in a glass-stoppered bottle, as it loses its strength rapidly if exposed to the air, and cork stoppers very
soon deteriorate. The fumes of ammonia are extremely irritating to the eyes, throat;
and nose, and particular care should be taken when opening bottles of it in hot weather, or when the bottles have been
left on a warm shelf, as the liquid may spurt out and cause serious damage. Bottles
containing liquor ammonia should be kept in a cool place, as heat develops great pressure, which may blow out the
stopper or burst the bottle. HAZARD very toxic, may
be fatal if swallowed. corrosive, it irritates the skin. Use gloves do not smell the bottle, the gas may cause shock
causing you to drop it. Ammonia Once diluted in the emulsion it is not corrosive or particularly hazardous, but
it must all be washed out to avoid fog or stains on coated material during development.
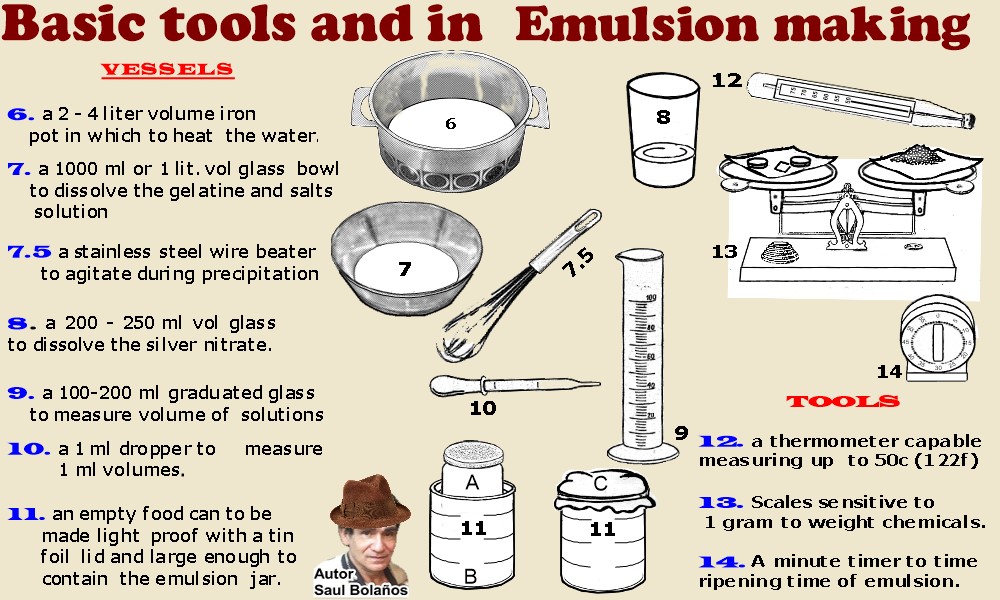
 Basic tools and
Basic tools and
chemicals in emulsion making
One of the most important tools Whatever type of heating disk or plate, it needs
adjusting the control knob to a minimum, and with the pot half filled with water on top; and a thermo-meter immersed in
it, ( fig 15) the water in pot observed for a long time, Rising or lowering the temp by careful movement of the knob,
until water comes to, and holds for hours an equilibrium temp. of 50c / 122 f. When this occurs, settings should not be
disturbed and should be marked by any means. -Do not place lid on pot, because heat will continue to rise
uncontrollably without ever reaching an equilibrium For coating at 35c/95f without disturbing the settings
see inside this book. A heating medium with exact and constant temperature control is absolutely essential for
good consistent emulsion manufacture.
Synopsis of Basic steps in emulsion making
By Normal Room Light
1) prepare salts gelatine solution
2) prepare silver solution
By Red Light
3) Precipitation: Add silver solution to Salts gelatine solution
4) First Ripening, hold mixed emulsion in a hot water bath
for the specified time and temperature when applicable.
5) Set Emulsion in ice water bath until it turns into a solid.
6) Shred or Break the solid emulsion to form noodles.
7) Gather in a Bag Place emulsion noodles inside a linen
bag for washing,
8) Wash Emulsion in ice water to get rid of the soluble
salts, secondary products of the reaction as nitrates
and excess of free bromides or ammonia if this last was
employed. (some emulsions do not need washing )
9) Melt Emulsion in hot water bath after washing
10) Second Ripening heat liquefied emulsion
(if applicable) for a specified time and temperature.
11) Coat Emulsion (still under red light) on paper or other
material and dry it for use. Store in total darkness.
NOTE The simplest and easiest emulsion to make is
Chloride Unwashed Positive Emulsion # 4. ( which see ) It is recomended that you make this emulsion before any
other given in this book. It uses no ammonia, it does not have to be washed, Can be made only using 3 basic
ingredients: 1. Ordinary Kitchen or table salt, 2.
gelatine and 3. Silver Nitrate. It furnishes a good emulsion.
DOWNLOAD MY CLASSIC BOOK:
" HOW TO MAKE PHOTOGRAPHIC PLATES AND PAPER"
 ABOVE, ONE OF MY MANY OTHER BOOKS ON PHOTOCHEMISTRY !
ABOVE, ONE OF MY MANY OTHER BOOKS ON PHOTOCHEMISTRY !
TEXT MESSAGES
IN
COSTA RICA
6012 4695
![]()
CONTACT

COSTA RICA © 2025, saul bolaños
CAFEDESAUL@GMAIL.COM